强生Zejula获美国FDA突破性药物资格中国大陆已进入优先审查!
2019-10-09 佚名 新浪医药
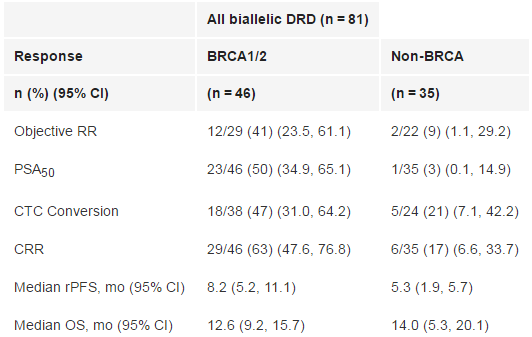
强生旗下杨森制药近日宣布,美国FDA已授予PARP抑制剂类抗癌药Zejula,用于治疗先前已接受紫杉烷化疗和雄激素受体(AR)靶向药物治疗、携带BRCA1/2基因突变的转移性去势抵抗性前列腺癌(mCRPC)患者。

本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言











#突破性药物资格#
46
#突破性药物#
43
#Zejula#
34
#药物资格#
39
#强生#
25
#优先审查#
29
#美国FDA#
32