欧盟,美国在加速审查诺华的CAR-T疗法Kymriah
2018-01-17 MedSci MedSci原创
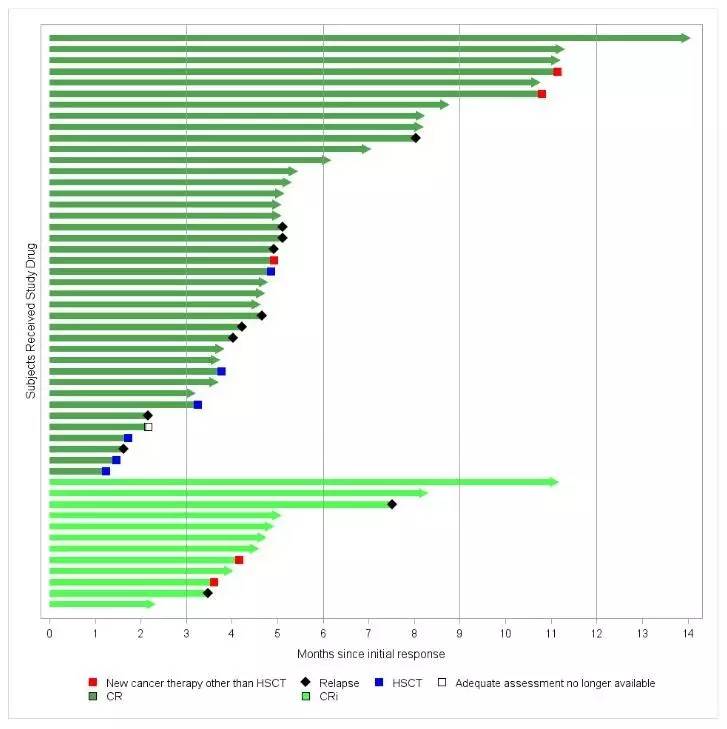
大西洋两岸的监管机构正在快速审查诺华的CAR-T疗法Kymriah(tisagenlecleucel;以前叫CTL019)。在美国,美国食品和药物管理局(FDA)已经批准该疗法的优先评估,以便使用该疗法来治疗不适合自体干细胞移植(ASCT)或ASCT复发的成年人。在欧盟,欧洲药物管理局已经对诺华公司的申请进行了加速评估,批准该疗法用于治疗儿童和年轻成人r/r B细胞急性淋巴细胞白血病和成人r/r
大西洋两岸的监管机构正在快速审查诺华的CAR-T疗法Kymriah(tisagenlecleucel;以前叫CTL019)。在美国,美国食品和药物管理局(FDA)已经批准该疗法的优先评估,以便使用该疗法来治疗不适合自体干细胞移植(ASCT)或ASCT复发的成年人。在欧盟,欧洲药物管理局已经对诺华公司的申请进行了加速评估,批准该疗法用于治疗儿童和年轻成人r/r B细胞急性淋巴细胞白血病和成人r/r DLBCL,他们不符合ASCT的要求。如果得到FDA和EMA的批准,Kymriah将代表第一个在非霍奇金淋巴瘤和B细胞中有两个明显迹象的CAR-T疗法。CAR-T提供了一种新的治疗方法,因为它是专门为每个病人制造的。 在此过程中,从患者的血液中提取T细胞,并在实验室中重新编程以创建基因编码的T细胞,以搜寻患者的癌细胞。Kymriah于去年8月获FDA批准用于治疗25岁以下患有B细胞前体ALL的难治性或第二次或以后复发的患者,成为第一个获得监管批准的CAR-T细胞疗法。诺华公司表示,其首项治疗显示该患者总体缓解率为83%,治疗方案有限,历史上结果不佳。为癌症治疗开辟新时代,诺华肿瘤全球药物开发部
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言











#CAR-#
36
#Kymriah#
40
#诺华#
30
在美国.美国食品和药物管理局(FDA)已经批准该疗法的优先评估.以便使用该疗法来治疗不适合自体干细胞移植(ASCT)或ASCT复发的成年人
59