一致性评价:37个品种被50%以上企业放弃,109个品种最被企业看重
2017-08-23 医药魔方 医药魔方
8月21日,CFDA对外公布了国内企业的一致性评价工作开展情况,主要是对289目录上被业选择评价和放弃评价的品种进行了统计(截至2017年5月23日)。从以下2点简单看一下这个数据:一、至少37个品种被50%以上的药企抛弃根据CFDA公布的统计结果,被列入289目录需要在2018年底前完成评价的品种中,以下36个品种被一半以上大药企放弃。考虑到统计时间滞后以及评价成功率等因素,289品种里面,被5
8月21日,CFDA对外公布了国内企业的一致性评价工作开展情况,主要是对289目录上被业选择评价和放弃评价的品种进行了统计(截至2017年5月23日)。从以下2点简单看一下这个数据:
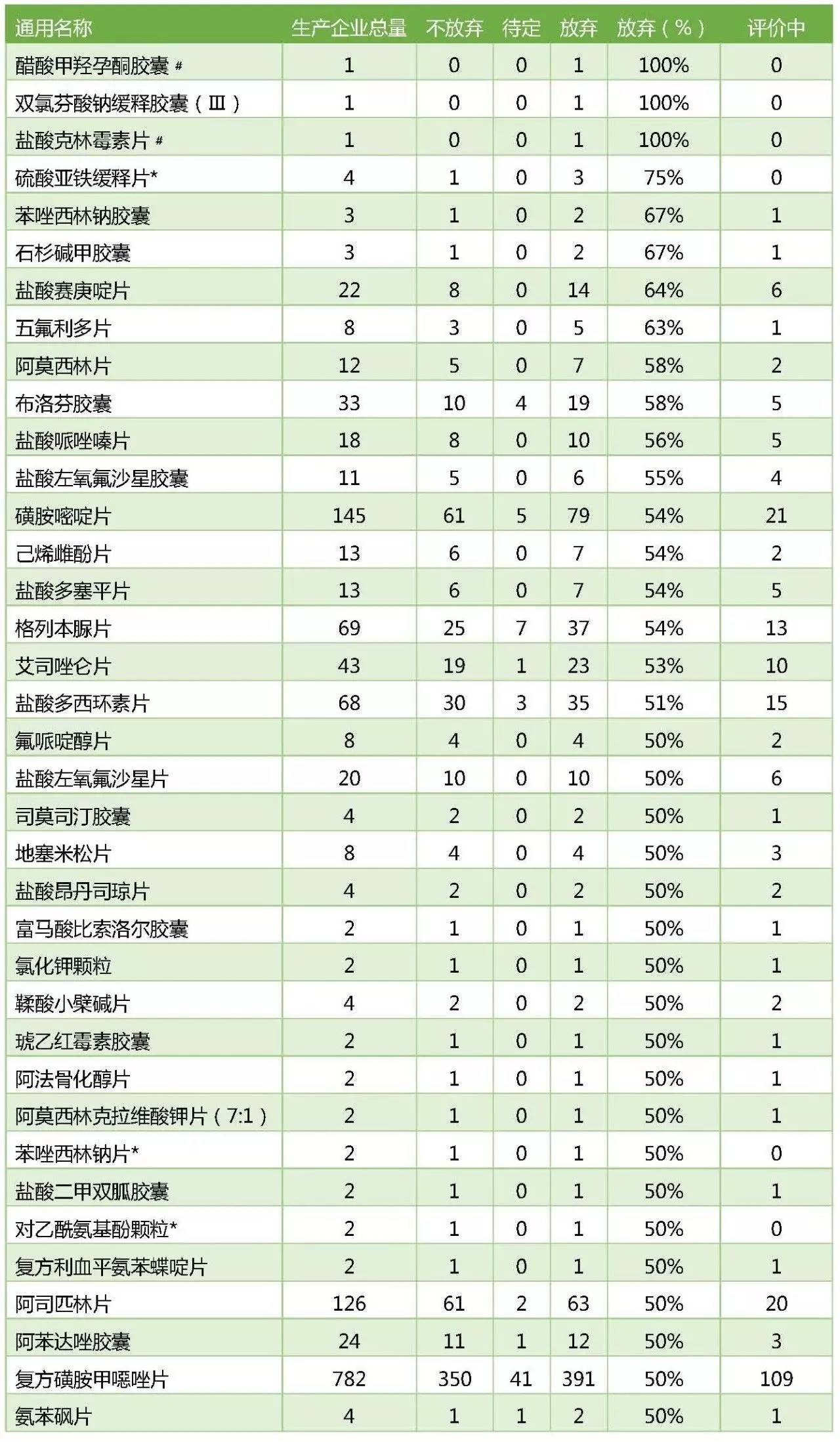
一、至少37个品种被50%以上的药企抛弃
根据CFDA公布的统计结果,被列入289目录需要在2018年底前完成评价的品种中,以下36个品种被一半以上大药企放弃。考虑到统计时间滞后以及评价成功率等因素,289品种里面,被50%以上企业放弃的品种预计也会远远超过37个。
在这37个品种中,磺胺嘧啶片、格列本脲片、阿司匹林片虽然也有很多企业未放弃,但真正开始评价的企业占比只有1/3左右。
50%以上企业放弃评价的品种
(表格中数字代表企业数量)
二、109个品种最被企业看重
有些品种被企业果断放弃,也有些品种被企业坚守不放,以下109个品种就被企业格外看重,超过80%的企业都表示不放弃。
这部分品种还有一个特点,企业比较果断,要么做,要么不做,很少出现企业待定犹豫不决的情况。比较有意思的一个品种是复方甘草片,36家生产企业,33家明确表示不放弃,但真正开始做的也就20家左右。
80%以上企业不愿放弃评价的品种
(表格中数字代表企业数量)


本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言











#一致性#
21
#评价#
17
#企业#
27
学习了.谢谢.
26