Ear Nose Throat J:持久过敏性鼻炎患者中氧化应激和硫醇/二硫化物平衡评估
2020-08-13 AlexYang MedSci原创
最近,有研究人员评估了药物治疗对硫醇/二硫化物平衡的影响,尽管过敏性刺激持续存在。
最近,有研究人员评估了药物治疗对硫醇/二硫化物平衡的影响,尽管过敏性刺激持续存在。

研究是一个前瞻性的观察研究,包括了35名持久过敏性鼻炎(AR)患者。研究人员对所有患者进行了皮刺测试,并使用Sino-nasal Outcome Test-22(SNOT-22)测试评估了鼻窦症状。研究人员使用了一种新型自动分光光度法测定硫醇/二硫化物稳态平衡参数,并进行统计学比较,并在治疗的第二个月测量了血清总硫醇(TT)、天然硫醇(SH)、二硫醇(SS)、二硫醇/天然硫醇(SS/SH)、二硫醇/总硫醇(SS/TT)、天然硫醇/总硫醇(SH/TT)比值。
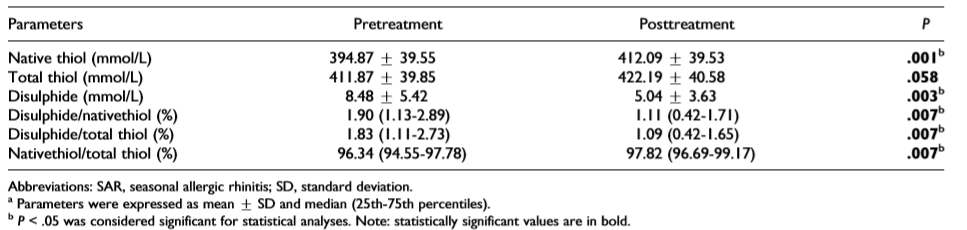
治疗前后血清硫醇值
参与研究的35名患者中,20名(58%)为女性。患者的平均年龄为33.17±9.9岁。治疗后,二硫化物、SS/SH和SS/TT比例显著下降(P<0.5),而SH和SH/TT的比例显著增加(P<0.05)。在第二个月SH测量均值显著增加(P=0.001),但TT测量均值在治疗后无明显变化(P=0.058)。SS测量值在治疗第二个月显著减少(P=0.003)。

SNOT-22差异与二硫化物关系
最后,研究人员指出,硫醇/二硫化物平衡可以作为持久AR治疗效果的标记。尽管处在过敏原接触中,治疗后SH水平的增加表明了氧化应激的减弱。
原始出处:
Ayse E Göker, Maide H Alagöz, Tolgar L Kumral et al. An Evaluation of Oxidative Stress With Thiol/Disulfide Homeostasis in Patients With Persistent Allergic Rhinitis. Ear Nose Throat J. Jul 2020
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言









#OAT#
25
#过敏性#
31
。
100
#应激#
30
学习
100