Nat Neurosci 外周巨噬细胞可能会延长ALS患者存活时间
2020-11-02 Shiqi Zhang MedSci原创
调节巨噬细胞具有影响疾病进展的能力,可能对ALS具有治疗价值。
小胶质细胞和外周巨噬细胞均与肌萎缩性侧索硬化症(ALS)有关,不过它们的各自作用尚待确定。现在该论文的研究显示,在小鼠ALS模型和ALS患者中,发现沿周围运动神经元轴突的巨噬细胞对神经退行性反应。在ALS小鼠模型中,使用细胞置换,对ALS小鼠外周骨髓细胞中活性氧物种途径进行靶向基因调节,降低了外周巨噬细胞和小胶质细胞的活化,发现症状延迟并能够提高存活率。转录组学研究表明,坐骨神经巨噬细胞和小胶质细胞对神经变性的反应不同,巨噬细胞的瞬时时间变化和小胶质细胞的进行性单向激活。修饰外周巨噬细胞抑制了促炎性小胶质细胞反应,并转向神经元支持。因此,调节巨噬细胞具有影响疾病进展的能力,可能对ALS具有治疗价值。
ALS是最常见的运动神经元(MN)疾病,其特征是上,下MN变性,导致进行性麻痹和患者死亡。脊髓运动神经元并不是常见的神经元,因为它们在脊髓内的体细胞被小胶质细胞包围,而其轴突(延伸到周围)被周围的巨噬细胞所包围。由于小胶质细胞(卵黄囊来源的)和外周巨噬细胞在不同环境中有不同作用,该研究推测它们可对ALS产生不同的作用。
该实验首先发现,在SOD1 G37R ALS小鼠模型和SOD1 G93AALS小鼠模型中,沿MN周围轴突的巨噬细胞被激活,下图中,进行ALS人类患者中小胶质细胞和外周巨噬细胞的活化,对具有腹侧(运动)和背侧(感觉)神经根和周围神经的脊髓进行了小胶质/巨噬细胞标记CD68的染色。发现CD68 +细胞存在于ALS脊髓腹角和腹根和ALS患者的周围神经,但对照组没有。
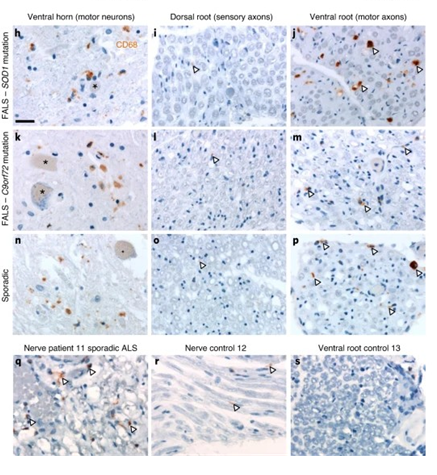
这里选择了ALS小鼠模型来评估外周神经巨噬细胞(独立于小胶质细胞)对疾病的影响能力,这里用具有降低的神经毒性ROS反应的巨噬细胞代替了(高效)突变型表达SOD1的外周巨噬细胞。在短期内,MN数量没有变化,在末期,MN数量只有微弱的改善。虽然这不足以增加存活率,但SOD1 WT / GFP BM移植能够增加肌肉强度。
接下来,发现周围神经巨噬细胞疾病发作时的置换可增加ALS小鼠的存活率,但在两组细胞移植的小鼠中,MN和CD3 +细胞数量没有差异,但在实验中观察到脊髓小胶质细胞激活减少,巨噬细胞活化降低,同时发现接受细胞移植的实验组小鼠存活率增加。因此,可以认为外围巨噬细胞替换的时机是巨噬细胞调节的关键参数,并且有可能通过修饰外围巨噬细胞来减慢疾病进程并增加ALS小鼠的存活率。
最后,使用RNA测序分析确定ALS小鼠在疾病过程中的坐骨神经末梢巨噬细胞反应。神经巨噬细胞调控的基因的热图分析表明,调控谱不是渐进的,而是突变的。在所分析的四个时间点中的每个时间点之间都有修改,包括在发作与早期症状之间以及早期症状与末期之间。小胶质细胞基因调控谱是单向的(大多从症状发生前开始上调)和进行性的。因此,巨噬细胞和小胶质细胞之间的基因调控模式极为不同。下图为神经巨噬细胞和小胶质细胞调控的基因的热图。
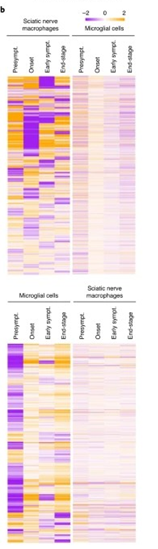
总之,修饰外周巨噬细胞可以下调神经周围和中枢神经系统的炎症,并且外周巨噬细胞可以影响疾病的进展和ALS小鼠模型的生存,因此认为调节外周巨噬细胞可能对ALS患者有治疗价值。
参考文献:
Aude Chiot, Sakina Zaïdi, et al. Modifying macrophages at the periphery has the capacity to change microglial reactivity and to extend ALS survival. Nature Neuroscience(2020) DOI:10.1038/s41593-020-00718-z
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言












#Nat#
27
#存活时间#
45
#ROS#
31
#学习#
89