Cancer Cell:肿瘤微环境影响前列腺癌耐药性
2020-07-28 Muse Fan MedSci原创
虽然第二代抗雄激素治疗药物已经显著提高了患者生存率,但即使联合使用最有效的AR通路抑制剂,依然有部分去势抵抗性前列腺癌患者无法获得完全缓解。
雄激素受体(androgen receptor,AR)信号通路在前列腺癌发生发展过程中起着核心作用。虽然第二代抗雄激素治疗药物已经显著提高了患者生存率,但即使联合使用最有效的AR通路抑制剂,依然有部分去势抵抗性前列腺癌(castration resistant prostate cancer, CRPC)患者无法获得完全缓解。目前国际各大临床指南对 CRPC 的定义不尽相同,主要是指前列腺癌患者经药物或手术去势治疗后,PSA仍持续升高和/或影像学发现前列腺癌进展。
因此了解经抗雄激素治疗后依然持续生长的肿瘤细胞的潜在生存机制至关重要。过往对于AR靶向治疗抵抗的研究都集中于探索肿瘤细胞内机制,但越来越多的研究证明肿瘤微环境也是耐药发生的重要驱动因素,如肿瘤微环境中的肿瘤相关成纤维细胞(cancer associated fibroblasts,CAFs)可通过细胞因子和蛋白酶调控肿瘤的血管生成,远处侵袭和抗凋亡等过程。
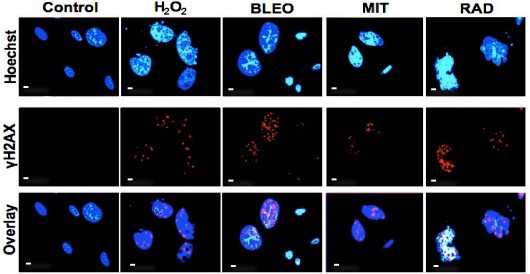
近日发表在《Cancer Cell》的一篇文章对肿瘤微环境在前列腺癌雄激素抵抗发生过程中的作用进行了研究。作者首先证明了CAFs细胞的培养液上清可以促进前列腺癌细胞在去雄激素环境下的生存,由此推断出CAFs可能分泌一种或多种可溶性因子,进而增加前列腺癌细胞的AR治疗抗性。进一步研究发现CAFs上清主要通过分泌性神经调节蛋白1(neuregulin 1, NRG1)结合肿瘤细胞的表皮生长因子受体3(human epidermal growth factor receptor3, HER3)来发挥作用,体内和体外实验都证明了阻断NRG1-HER3轴可以有效抑制肿瘤细胞对于抗雄激素治疗的耐药性。

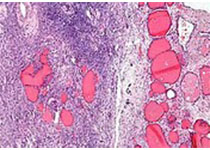
新辅助内分泌治疗是指根治性前列腺癌切除术前进行的包括药物去势和抗雄激素治疗等在内的内分泌治疗,可减少肿瘤体积,降低临床分期。如上图所示,该研究对43例进行前列腺癌根治性切除术的病人病理组织进行分析发现,23例术前进行新辅助内分泌治疗的病人有5例NRG1表达阳性,而术前未接受内分泌治疗的20例病人中NRG1表达均为阴性。

作者进一步对有治疗结果记录的一个CRPC队列患者进行分析,发现NRG1的活性与治疗预后呈负相关,NRG1活性低的患者经过抗雄激素治疗后生存时间更长。
研究得出:CAFs来源的NRG1可以促进前列腺癌抗雄激素治疗的耐药性,药物性地阻断NRG1-HER3轴可以促进抑制前列腺癌患者激素抵抗的发生。NRG1在经抗雄激素治疗后的前列腺癌基质中表达升高,高活性的NRG1会抑制CRPC患者对第二代抗雄激素治疗药物的敏感性。该研究为未来CRPC的机制探索和治疗方案提供了新的思路和靶点。
文章出处:Zhang Z, Karthaus WR, Lee YS, Gao VR, Wu C, Russo JW, et al. Tumor Microenvironment-Derived NRG1 Promotes Antiandrogen Resistance in Prostate Cancer. Cancer Cell 2020.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言








#cancer cell#
28
#CEL#
29
#Cell#
36
该研究为未来CRPC的机制探索和治疗方案提供了新的思路和靶点。
2
前列腺癌相关研究,学习了,谢谢梅斯
39
受益匪浅
76