DIA2017丨CFDA专场:监管改革推动药品质量和创新
2017-05-25 林云 DIA订阅号
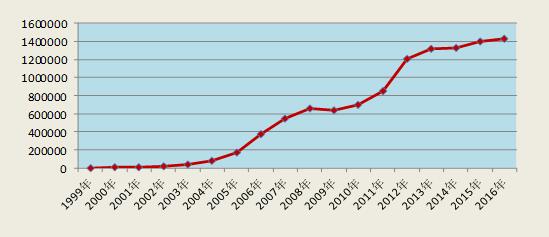
昨天下午举行的2017中国国际药物信息大会暨第九届DIA中国年会CFDA专场上由中国食品药品国际交流中心的薛斌主任和DIA中国董事总经理朱立红女士担任主持人,总局药化监管司的李金菊副司长、中检院的萧红街先生、总局药品审评中心的黄清竹先生和总局食品药品审核查验中心的董江萍副主任分别作了演讲。之后,李金菊、黄清竹、萧红街和中检院副院长张志军共同在讨论环节回答了参会者提出的十多个问题。审评审批制度改革2
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言












#药品质量#
35
#CFDA#
25
#DIA#
30
#创新#
27
#监管#
30