药审中心拟优先审评名单更新
2019-04-22 佚名 中国药审
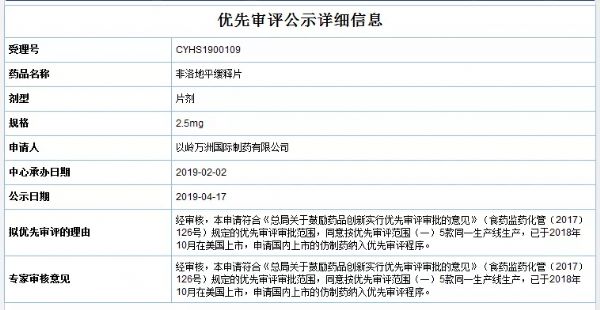
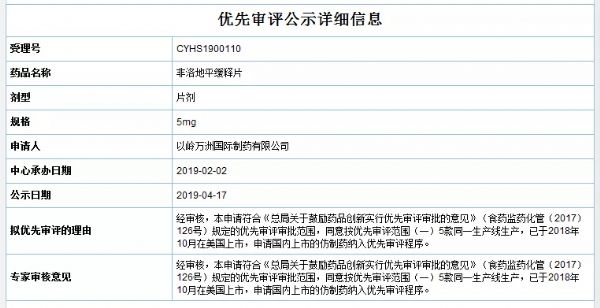
4月17日,药审中心公示的拟优先审评的药品品种新增以岭万洲国际制药有限公司的非洛地平缓释片(规格为2.5mg、5mg、10mg),公示期间如有异议,可在药审中心网站“信息公开-->优先审评公示-->拟优先审评品种公示”栏目下提出异议。
4月17日,药审中心公示的拟优先审评的药品品种新增以岭万洲国际制药有限公司的非洛地平缓释片(规格为2.5mg、5mg、10mg),公示期间如有异议,可在药审中心网站“信息公开-->优先审评公示-->拟优先审评品种公示”栏目下提出异议。
拟优先审评品种公示名单

版权声明:
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言









#优先审评#
31
#药审#
48
#药审中心#
36