Alzheimer&Dementia:大脑氧提取分数变化与血管和阿尔茨海默病的关系
2022-07-10 MedSci原创 MedSci原创
由此可见,老年人的OEF纵向变化主要与血管病变有关。
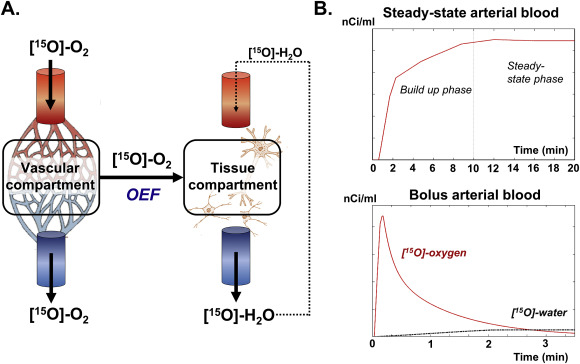
脑氧提取分数(OEF)是大脑氧消耗和供应的重要生理指标,已被认为是中风、阿尔茨海默病、多发性硬化症、镰状细胞病和代谢紊乱等多种疾病的潜在生物标志物。然而,为了使氧气提取分数成为个性化疾病诊断的敏感生物标志物,必须最小化或考虑正常受试者的受试者间差异,否则这将掩盖其解释。
近期,发表在Alzheimer&Dementia杂志上的研究纵向测量了老年人的OEF,以研究其与阿尔茨海默病(AD)和血管病变标志物的关系。

研究人员对137名参与者进行了两个时间点的研究,间隔时间为2.16年。使用T2松弛-下旋标记(TRUST)磁共振成像(MRI)测量OEF。研究了OEF与血管风险、白质增生(WMH)、脑脊液(CSF)中淀粉样β(Aβ)、总tau(t-tau)和磷酸化tau181(p-tau181)之间的关系。
结果显示,从基线到随访,OEF有所增加。与低血管风险者相比,高血管风险者的OEF增加更为突出,并与血管风险的发展和WMH体积的增加有关。OEF的变化与AD病理的CSF标志物或其进展无关。
由此可见,老年人的OEF纵向变化主要与血管病变有关。
参考文献:
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言















#阿尔茨#
35
#阿尔茨海#
41
#阿尔茨海默#
33
#dementia#
39