CGP综述|肌肉减少性肥胖在癌症中的研究进展
2024-01-03 中国全科医学杂志 中国全科医学杂志 发表于上海
本文总结文献得出,SO在癌症患者中发生率较高,但目前对于其定义及诊断标准仍具有争议。SO是癌症预后的独立预测因素,具有重要临床应用价值。
本文来源
彭磊,朱克祥. 肌肉减少性肥胖在癌症中的研究进展[J]. 中国全科医学, 2024, 27(06): 643-649.
随着世界范围内肥胖和老年人口的增加,肌肉减少性肥胖(SO)正在成为与多种临床环境(包括癌症)中不良事件和结局风险较高的相关因素。但目前对于SO的定义及诊断缺乏统一标准,且其与癌症之间的相互作用关系有待进一步阐明。本文系统、全面地总结了SO的相关定义及诊断方法,将其对癌症患者的临床影响展开具体论述,包括对手术及化疗患者的影响,并总结其主要防治策略。
本文总结文献得出,SO在癌症患者中发生率较高,但目前对于其定义及诊断标准仍具有争议。SO是癌症预后的独立预测因素,具有重要临床应用价值。
01 SO的定义及诊断标准
定义:根据ESPEN和EASO发布的《肌肉减少性肥胖的定义和诊断标准共识》,SO被定义为一种肌肉减少症和肥胖并存的临床和功能性疾病,其特征是脂肪含量高和骨骼肌质量降低及骨骼肌功能低下。
诊断标准:目前SO的诊断方法尚无统一标准,现有文献主要有两种方法,一种是分别对肌肉减少症和肥胖两种疾病单独自主定义,当两者并存时诊断为SO。根据先前的研究,通过患者的CT图像计算L3水平的总骨骼肌组织面积(TAMA),并将TAMA除以身高的平方得到骨骼肌指数(SMI)作为评估肌肉减少症的指标。并且由于男性和女性身体成分存在显著差异,提出了定义肌肉减少症的性别特异性SMI临界值(西方人群:男性和女性分别为52.4、38.5 cm2/m2;东方人群:男性和女性分别为36.2、29.6 cm2/m2)。肥胖的临界值也有所差异,大多数研究将BMI>25 kg/m2定义为肥胖,部分研究中的临界值设定BMI>30 kg/m2。此外,一些研究定义了自己的临界值。
另一种诊断方法则是将L3水平的VFA与TAMA的比率作为诊断SO的单独指标,该比率可以在不考虑总体质量的情况下强调不成比例的内脏脂肪含量和肌肉质量。虽然目前在脂肪量测量方面没有方法论上的共识,但与癌症患者的高脂肪量(内脏肥胖)相比,BMI与长期结局和预后之间的关联较弱。因此,越来越多的研究在定义癌症患者的SO时引入了脂肪量或内脏脂肪量,而不是通过BMI进行评估。
02 SO与癌症之间的相互作用机制
SO的发病因素复杂,包括衰老、不当的生活方式(久坐不动、饮食不当、缺乏运动)、炎症、急慢性疾病和癌症等合并症。同时,肌肉质量的减少及功能的丧失与脂肪蓄积的双重压力也会导致癌症患者出现虚弱、残疾、代谢性疾病、术后并发症、化疗剂量限制毒性等并发症,从而导致患者的不良预后。
癌症与SO的相互作用机制目前尚不明确,以下几个因素可能导致进行性的脂肪代谢和骨骼肌改变,从而导致PC患者中的SO,如图1所示。
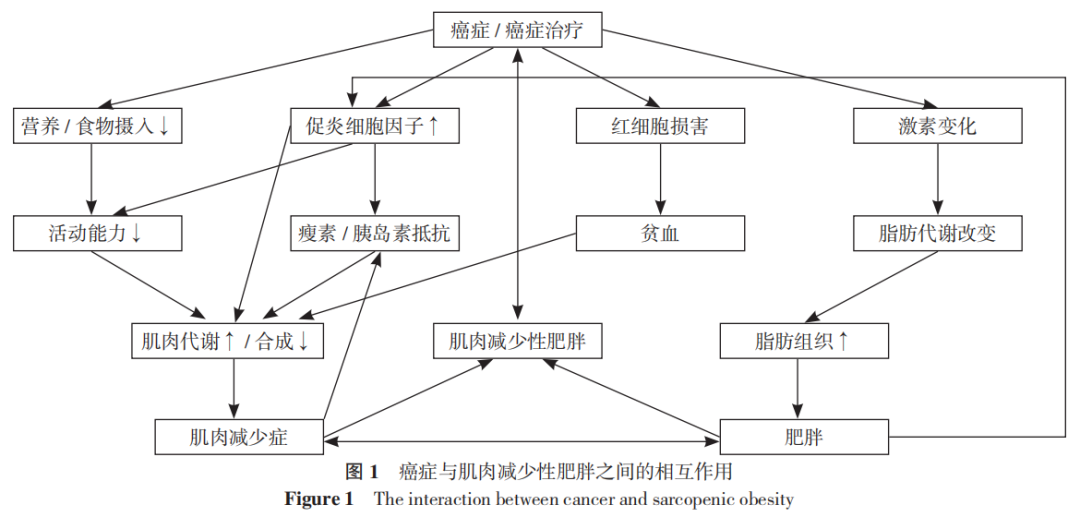
03 SO对癌症患者的临床影响
3.1 SO对术后并发症的影响
近年来越来越多的研究证实了SO对癌症患者预后不良的影响,并且是术后主要并发症的有效预测指标。PECORELLI等分析了202例接受胰腺手术的患者,发现SO(VFA/TAMA>3.2)与术后死亡及胰瘘风险显著相关。YAMANE等也发现与非SO者相比,SO患者的ISGPF B/C级胰瘘发生率显著增加。SANDINI等对124例胰腺癌患者进行的回顾性分析也证实了SO对主要手术并发症风险的预测作用:在多变量分析中,VFA和TAMA之间的比率是主要并发症的最强预测指标,优势比为3.20。
3.2 SO对化疗毒性的影响
多项研究已经证明,化疗可以改变身体成分,减少骨骼肌质量及功能,促进SO的发展,从而对化疗的耐受性产生显著影响,并且可能会产生更多的不良反应。PRADO等研究表明,在用5-氟尿嘧啶和亚叶酸钙治疗结肠癌的患者中,肌肉减少症是剂量限制性毒性的重要预测指标。KURITA等发表的一项关于FOLFIRINOX(奥沙利铂、伊立替康、亚叶酸钙和氟尿嘧啶)治疗胰腺癌的研究显示,SO与高级别血液学毒性风险增加相关。因此,对于SO的癌症患者,最好适当减少化疗药物的剂量,以降低化疗毒性及相关不良反应的发生。
3.3 肥胖与肌肉减少症对癌症患者免疫治疗的影响
3.3.1 肥胖对免疫疗法疗效的影响:
肥胖和癌症免疫治疗之间的关系很大程度上与胰岛素抵抗、性激素升高、脂肪因子分泌调节和PD-1表达上调有关。一项纳入了976例接受抗PD-1/PD-L1免疫治疗的晚期癌症患者的回顾性横向研究发现肥胖(BMI≥25 kg/m2)与改善ICIs的临床结局之间存在显著相关性,为了验证这一结论,该研究团队构建了肥胖小鼠的临床前模型,证实肥胖小鼠的T细胞功能障碍部分由PD-1轴介导,并由瘦素驱动,这加强了非受体酪氨酸激酶/信号转导子和转录激活子(JAK/STAT)通路与免疫检查点抑制之间的已知相关性。另一项独立研究发现,在非小细胞肺癌(NSCLC)患者中,使用抗PD-L1治疗的患者BMI增加与总生存率之间存在线性关联,特别是BMI≥30kg/m2的患者的总生存率显著提高。
3.3.2 肌肉减少症对免疫疗法疗效的影响:
肌肉减少症目前已被强调为PD-1抑制剂免疫治疗的癌症患者预后不良的重要预测因素。对于肌肉减少症与免疫治疗预后差之间的关系,目前认为主要有以下因素:首先,慢性炎症是导致肌肉减少症的主要原因,它导致肿瘤细胞免疫逃逸,如T细胞耗竭;其次,一些可能参与肌肉减少症发生的生物标志物[如组织生长因子β(TGF-β)和IL-6]削弱了肿瘤对免疫检查点抑制剂反应。因此,测量肌肉减少症可能有助于识别癌症患者的各种类型,使癌症患者能够受益于免疫治疗。
3.4 SO对生存期的影响
多项研究已经证明,对于可手术和不可手术的癌症患者,SO均与较短的OS正相关。在一项专门针对OS的研究中,SO是胃肠道(胃癌、胰腺癌等)和呼吸道癌症患者OS的重要预测因素。另外,一项分析了465例因肝细胞癌接受原发性肝切除术患者的回顾性研究发现,SO患者的中位生存期和中位无复发生存期均较差。CHARGI等的研究也证明了SO是OS和无病生存的负面预后因素。
04 SO防治策略
4.1 营养治疗
最近,一项针对恶病质的临床试验证实了营养治疗的重要性,通过营养补充可以维持或增加骨骼肌质量,以改善SO癌症患者的预后以及生活质量。事实上,保持适当的营养状况,可以帮助恢复肌肉质量和功能,从而减少患有SO的癌症患者的术后并发症发病率和死亡率,这可能是防治SO的潜在策略。
一项随机对照试验显示,在社区居住的老年男性中,足够的营养支持(乳清蛋白、维生素D和钙)与肌肉质量和功能的改善有关。PRADO等最近的评论也讨论了营养治疗在预防和逆转癌症患者肌肉减少症中的作用,这种方法也可能适用于SO。研究证明在癌症患者中保留了足够的肌肉蛋白合成代谢潜能,蛋白质摄入时间会影响肌肉蛋白质合成:一项针对年轻人的研究评估,与蛋白质分布不平衡相比,全天恒定的蛋白质摄入量可增强每日肌肉蛋白质的合成。尽管营养治疗似乎与SO患者临床结局的改善可能相关,但尚未进行任何干预研究,缺乏具体证据。
4.2 运动疗法
除了营养干预外,运动疗法也可能是逆转SO的关键策略。阻力训练和一般运动干预已被证明可以改善肌肉质量和功能,同时减少脂肪组织的蓄积,从而在一定程度上减少SO给癌症患者带来的负面影响。虽然由于各种原因(包括乏力和癌症相关的疼痛),运动疗法可能对癌症患者具有挑战性,但越来越多的证据强调了运动训练在恢复骨骼肌质量和功能及减少脂肪组织蓄积的癌症环境中一些好处。因此,未来的研究应着重研究其本身或与营养治疗相结合的潜在临床相关性,特别是对于患有SO的癌症患者。
05 小结
尽管目前在定义和诊断标准上仍缺乏共识,但全球越来越多的证据表明,SO是癌症患者中重要的临床相关性的因素。通过身体成分评估进行的化学疗法剂量计算,可能有助于减少与治疗相关的毒性,并最终改善患者的预后。而且,基于CT图像的身体成分评估可以很容易地应用于临床环境,可能有助于识别那些预后较差的癌症患者,从而在早期通过营养或运动疗法进行干预,减少患者骨骼肌的丢失和脂肪组织的蓄积,改善患者的临床预后甚至在一定程度上提高生存率。
参考文献
[1] WU C C,LI M N,MENG H B,et al. Analysis of status and countermeasures of cancer incidence and mortality in China[J]. Sci China Life Sci,2019,62(5):640-647.
[2] TAKEDA T,SASAKI T,OKAMOTO T,et al. Prognostic impact of osteosarcopenia in patients with advanced pancreatic cancer receiving gemcitabine plus nab-paclitaxel[J]. Pancreatology,2023,23(3):275-282.
[3] CRUZ-JENTOFT A J,BAHAT G,BAUER J,et al. Sarcopenia:revised European consensus on definition and diagnosis[J]. Age Ageing,2019,48(4):601.
[4] CHAN M Y,CHOK K S H. Sarcopenia in pancreatic cancer - effects on surgical outcomes and chemotherapy[J]. World J Gastrointest Oncol,2019,11(7):527-537.
[5] ROUBENOFF R. Excess baggage:sarcopenia,obesity,and cancer outcomes[J]. Lancet Oncol,2008,9(7):605-607.
[6] SANDINI M,BERNASCONI D P,FIOR D,et al. A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer[J]. Nutrition,2016,32(11/12):1231-1237.
[7] PECORELLI N,CARRARA G,DE COBELLI F,et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery[J]. Br J Surg,2016,103(4):434-442.
[8] KIM Y M,KIM J H,BAIK S J,et al. Sarcopenia and sarcopenic obesity as novel risk factors for gastric carcinogenesis:a health checkup cohort study[J]. Front Oncol,2019,9:1249.
[9] CARNEIRO I P,MAZURAK V C,PRADO C M. Clinical implications of sarcopenic obesity in cancer[J]. Curr Oncol Rep,2016,18(10):62.
[10] DONINI L M,BUSETTO L,BISCHOFF S C,et al. Definition and diagnostic criteria for sarcopenic obesity:ESPEN and EASO consensus statement[J]. Obes Facts,2022,15(3):321-335.
[11] BARACOS V E,ARRIBAS L. Sarcopenic obesity:hidden muscle wasting and its impact for survival and complications of cancer therapy[J]. Ann Oncol,2018,29(Suppl 2):ii1-9.
[12] MIJNARENDS D M,MEIJERS J M M,HALFENS R J G,et al. Validity and reliability of tools to measure muscle mass,strength,and physical performance in community-dwelling older people:a systematic review[J]. J Am Med Dir Assoc,2013,14(3):170-178.
[13] FARON A,SPRINKART A M,KUETTING D L R,et al. Body composition analysis using CT and MRI:intra-individual intermodal comparison of muscle mass and myosteatosis[J]. Sci Rep,2020,10(1):11765.
[14] MOURTZAKIS M,PRADO C M M,LIEFFERS J R,et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care[J]. Appl Physiol Nutr Metab,2008,33(5):997-1006.
[15] MONTANO-LOZA A J,ANGULO P,MEZA-JUNCO J,et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis[J]. J Cachexia Sarcopenia Muscle,2016,7(2):126-135.
[16] PRADO C M M,LIEFFERS J R,MCCARGAR L J,et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts:a population-based study[J]. Lancet Oncol,2008,9(7):629-635.
[17] CHO W K,YU J I,PARK H C,et al. Impact of sarcopenia on survival of pancreatic cancer patients treated with concurrent chemoradiotherapy[J]. Tumori,2021,107(3):247-253.
[18] SUGAWARA K,YAMASHITA H,OKUMURA Y,et al. Relationships among body composition,muscle strength,and sarcopenia in esophageal squamous cell carcinoma patients[J]. Support Care Cancer,2020,28(6):2797-2803.
[19] JANG M,PARK H W,HUH J,et al. Predictive value of sarcopenia and visceral obesity for postoperative pancreatic fistula after pancreaticoduodenectomy analyzed on clinically acquired CT and MRI[J]. Eur Radiol,2019,29(5):2417-2425.
[20] RICKLES A S,IANNUZZI J C,MIRONOV O,et al. Visceral obesity and colorectal cancer:are we missing the boat with BMI?[J]. J Gastrointest Surg,2013,17(1):133-143;discussionp.143.
[21] KIMURA Y,YAMADA M,OHJI S,et al. Presence of sarcopenic obesity and evaluation of the associated muscle quality in Japanese older men with prostate cancer undergoing androgen deprivation therapy[J]. J Geriatr Oncol,2019,10(5):835-838.
[22] YAMANE H,ABE T,AMANO H,et al. Visceral adipose tissue and skeletal muscle index distribution predicts severe pancreatic fistula development after pancreaticoduodenectomy[J]. Anticancer Res,2018,38(2):1061-1066.
[23] BATSIS J A,VILLAREAL D T. Sarcopenic obesity in older adults:aetiology,epidemiology and treatment strategies[J]. Nat Rev Endocrinol,2018,14(9):513-537.
[24] FACCIORUSSO A,ANTONINO M,MUSCATIELLO N. Sarcopenia represents a negative prognostic factor in pancreatic cancer patients undergoing EUS celiac plexus neurolysis[J]. Endosc Ultrasound,2020,9(4):238-244.
[25] KHAN I M,PERRARD X Y,BRUNNER G,et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance[J]. Int J Obes (Lond),2015,39(11):1607-1618.
[26] BHULLAR A S,ANOVEROS-BARRERA A,DUNICHAND-HOEDL A,et al. Lipid is heterogeneously distributed in muscle and associates with low radiodensity in cancer patients[J]. J Cachexia Sarcopenia Muscle,2020,11(3):735-747.
[27] MINTZIRAS I,MILIGKOS M,WÄCHTER S,et al. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer:systematic review and meta-analysis[J]. Int J Surg,2018,59:19-26.
[28] BIOLO G,CEDERHOLM T,MUSCARITOLI M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases:from sarcopenic obesity to cachexia[J]. Clin Nutr,2014,33(5):737-748.
[29] DOYLE S L,LYSAGHT J,REYNOLDS J V. Obesity and post-operative complications in patients undergoing non-bariatric surgery[J]. Obes Rev,2010,11(12):875-886.
[30] REISINGER K W,DERIKX J P M,VAN VUGT J L A,et al. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer[J]. Clin Nutr,2016,35(4):924-927.
[31] BEAVERS K M,HSU F C,HOUSTON D K,et al. The role of metabolic syndrome,adiposity,and inflammation in physical performance in the Health ABC Study[J]. J Gerontol A Biol Sci Med Sci,2013,68(5):617-623.
[32] 国家卫生健康委员会. 胰腺癌诊疗规范(2018年版)[J].中华消化病与影像杂志(电子版),2019,9(5):224-240.
[33] ARGILÉS J M,BUSQUETS S,STEMMLER B,et al. Cachexia and sarcopenia:mechanisms and potential targets for intervention[J]. Curr Opin Pharmacol,2015,22:100-106.
[34] TILG H,MOSCHEN A R. Adipocytokines:mediators linking adipose tissue,inflammation and immunity[J]. Nat Rev Immunol,2006,6(10):772-783.
[35] SATO H,CARVALHO G,SATO T,et al. The association of preoperative glycemic control,intraoperative insulin sensitivity,and outcomes after cardiac surgery[J]. J Clin Endocrinol Metab,2010,95(9):4338-4344.
[36] KURITA Y,KOBAYASHI N,TOKUHISA M,et al. Sarcopenia is a reliable prognostic factor in patients with advanced pancreatic cancer receiving FOLFIRINOX chemotherapy[J]. Pancreatology,2019,19(1):127-135.
[37] COUSIN S,HOLLEBECQUE A,KOSCIELNY S,et al. Low skeletal muscle is associated with toxicity in patients included in phase I trials[J]. Invest New Drugs,2014,32(2):382-387.
[38] YIP C,GOH V,DAVIES A,et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer[J]. Eur Radiol,2014,24(5):998-1005.
[39] PRADO C M M,BARACOS V E,MCCARGAR L J,et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity[J]. Clin Cancer Res,2007,13(11):3264-3268.
[40] EMORI T,ITONAGA M,ASHIDA R,et al. Impact of sarcopenia on prediction of progression-free survival and overall survival of patients with pancreatic ductal adenocarcinoma receiving first-line gemcitabine and nab-paclitaxel chemotherapy[J]. Pancreatology,2022,22(2):277-285.
[41] TSAI S. Importance of lean body mass in the oncologic patient[J]. Nutr Clin Pract,2012,27(5):593-598.
[42] YOUN S,CHEN A,HA V,et al. An exploratory study of body composition as a predictor of dose-limiting toxicity in metastatic pancreatic cancer treated with gemcitabine plus nab-paclitaxel[J]. Clin Nutr,2021,40(8):4888-4892.
[43] BAXEVANIS C N,PEREZ S A,PAPAMICHAIL M. Cancer immunotherapy[J]. Crit Rev Clin Lab Sci,2009,46(4):167-189.
[44] YOUSEFI H,YUAN J D,KESHAVARZ-FATHI M,et al. Immunotherapy of cancers comes of age[J]. Expert Rev Clin Immunol,2017,13(10):1001-1015.
[45] CHENG A L,HSU C,CHAN S L,et al. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma[J]. J Hepatol,2020,72(2):307-319.
[46] PASQUARELLI-DO-NASCIMENTO G,MACHADO S A,DE CARVALHO J M A,et al. Obesity and adipose tissue impact on T-cell response and cancer immune checkpoint blockade therapy[J]. Immunother Adv,2022,2(1):ltac015.
[47] CLOUGHESY T F,MOCHIZUKI A Y,ORPILLA J R,et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma[J]. Nat Med,2019,25(3):477-486.
[48] CORTELLINI A,BERSANELLI M,BUTI S,et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors:when overweight becomes favorable[J]. J Immunother Cancer,2019,7(1):57.
[49] WANG Z M,AGUILAR E G,LUNA J I,et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade[J]. Nat Med,2019,25(1):141-151.
[50] MULLEN M,GONZALEZ-PEREZ R R. Leptin-induced JAK/STAT signaling and cancer growth[J]. Vaccines,2016,4(3):26.
[51] KICHENADASSE G,MINERS J O,MANGONI A A,et al. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer[J]. JAMA Oncol,2020,6(4):512-518.
[52] CORTELLINI A,BOZZETTI F,PALUMBO P,et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors:a multicenter real-life study[J]. Sci Rep,2020,10(1):1456.
[53] DALY L E,POWER D G,O'REILLY A,et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma[J]. Br J Cancer,2017,116(3):310-317.
[54] GUO Y S,REN Y Q,WU F H,et al. Prognostic impact of sarcopenia in patients with hepatocellular carcinoma treated with PD-1 inhibitor[J]. Therap Adv Gastroenterol,2022,15:17562848221142417.
[55] KANO M,HIHARA J,TOKUMOTO N,et al. Association between skeletal muscle loss and the response to nivolumab immunotherapy in advanced gastric cancer patients[J]. Int J Clin Oncol,2021,26(3):523-531.
[56] SHIROYAMA T,NAGATOMO I,KOYAMA S,et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors:a preliminary retrospective study[J]. Sci Rep,2019,9(1):2447.
[57] TOZUKA Y,UENO M,KOBAYASHI S,et al. Prognostic significance of sarcopenia as determined by bioelectrical impedance analysis in patients with advanced pancreatic cancer receiving gemcitabine plus nab-paclitaxel:a retrospective study[J]. Oncol Lett,2022,24(4):375.
[58] LODEWICK T M,VAN NIJNATTEN T J A,VAN DAM R M,et al. Are sarcopenia,obesity and sarcopenic obesity predictive of outcome in patients with colorectal liver metastases?[J]. HPB,2015,17(5):438-446.
[59] KOBAYASHI A,KAIDO T,HAMAGUCHI Y,et al. Impact of sarcopenic obesity on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma[J]. Ann Surg,2019,269(5):924-931.
[60] CHARGI N,BRIL S I,SWARTZ J E,et al. Skeletal muscle mass is an imaging biomarker for decreased survival in patients with oropharyngeal squamous cell carcinoma[J]. Oral Oncol,2020,101:104519.
[61] SOLHEIM T S,LAIRD B J A,BALSTAD T R,et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer[J]. J Cachexia Sarcopenia Muscle,2017,8(5):778-788.
[62] RICHTER E,DENECKE A,KLAPDOR S,et al. Parenteral nutrition support for patients with pancreatic cancer—improvement of the nutritional status and the therapeutic outcome[J]. Anticancer Res,2012,32(5):2111-2118.
[63] KEMMLER W,KOHL M,FRÖHLICH M,et al. Effects of high-intensity resistance training on osteopenia and sarcopenia parameters in older men with osteosarcopenia-one-year results of the randomized controlled Franconian osteopenia and sarcopenia trial (FrOST)[J]. J Bone Miner Res,2020,35(9):1634-1644.
[64] PRADO C M,PURCELL S A,LAVIANO A. Nutrition interventions to treat low muscle mass in cancer[J]. J Cachexia Sarcopenia Muscle,2020,11(2):366-380.
[65] WITARD O C,WARDLE S L,MACNAUGHTON L S,et al. Protein considerations for optimising skeletal muscle mass in healthy young and older adults[J]. Nutrients,2016,8(4):181.
[66] FLOREZ BEDOYA C A,CARDOSO A C F,PARKER N,et al. Exercise during preoperative therapy increases tumor vascularity in pancreatic tumor patients[J]. Sci Rep,2019,9(1):13966.
[67] KIRK B,ZANKER J,DUQUE G. Osteosarcopenia:epidemiology,diagnosis,and treatment-facts and numbers[J]. J Cachexia Sarcopenia Muscle,2020,11(3):609-618.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言













#癌症# #肌肉减少性肥胖#
33