自噬--细胞的守护神(下)
2014-02-25 MedSci MedSci原创
9. Exercise can both activate and inhibit autophagy. For this reason, the benefits of exercise are mostly due to non-autophagy factors. Decreased autophagy mechanisms with
9. Exercise can both activate and inhibit autophagy.
For this reason, the benefits of exercise are mostly due to non-autophagy factors.
Decreased autophagy mechanisms with exercise: Exercise up regulates mTOR, especially resistance exercises like weight lifting. Exercise also activates the IGF-1 pathway by increasing growth hormone secretion by the pituitary gland, which then in turn stimulates IGF-1 production by the liver. IGF-1 inhibits autophagy via the Insulin/IGF-1/PI3K/Akt pathway.
Increased autophagy mechanisms with exercise: ROS increases with exercise. Since ROS activates autophagy, this is one mechanism by which exercise could activate autophagy, but it is unclear if this activates “selective mitochondrial destruction” this way (i.e. mitophagy).
Hypoxia also activates autophagy via a HIF-1a pathway. This would occur with exercise if you reached your anaerobic threshold during exercise or did IHT exercise (intermittent hypoxia with exercise).
Conclusion: Exercise can both inhibit and activate autophagy. This may be why it is difficult to show exactly how exercise prolongs lifespan.
10. Autophagy exercises anti-aging effects on postmitotic cells.
- There are primarily 5 cytoprotective effects:
- Reduced accumulation of toxic protein aggregates, described above
- Destroying bad mitochondria via mitophagy, described above
- Reduced apoptosis
- Reduced necrosis
- Improved hormesis
Cells that do not divide are particularly vulnerable to the build-up of protein aggregates seen in neurodegenerative diseases. Autophagy inducers such as rapamycin, rapalogs, valproate, and lithium have been shown to help in experimental models of Huntington’s disease, tauopathies, Alzheimer’s disease, and Parkinson’s disease.
When mitochondria are defective due to ROS-induced damage, asymmetric fission occurs, allowing for a good mitochondria and a bad mitochondria to “split up”. The bad mitochondria has a low membrane potential and is tagged by PINK1 and then ubiquinated by Parkin. At this point, it is recognized by the autophagy system and is destroyed by macroautophagy.
Autophagy also has an anti-apoptotic function in post mitotic cells. Autophagy helps damaged cells recover and thereby avoid apoptosis. Autophagy also has an “anti-necrosis” function in post mitotic cells.
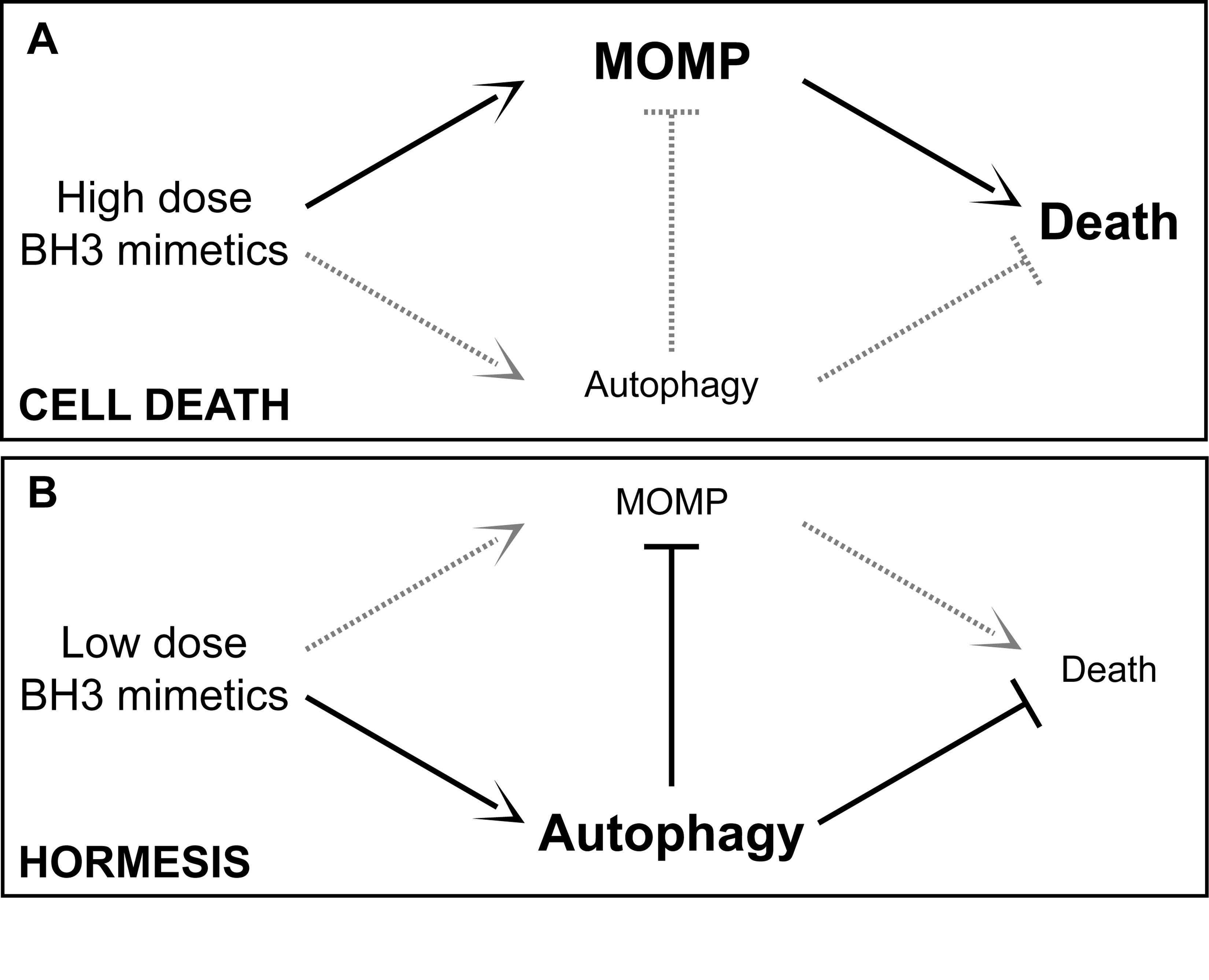
Autophagy is also a stress response involving hormesis. Hormesis is how low (sublethal) doses of cellular stressors result in an up regulation of cellular stress adaptation mechanisms. See the blog entries Multifactorial hormesis II – Powerpoint presentation and Multifactorial Hormesis – the theory and practice of maintaining health and longevity. Autophagy has a hormetic dose response curve. Depending on the strength or duration of the stressor, autophagy or a negative consequence could ensue, as exemplified in this diagram:
11. Anti-aging effects of Autophagy on Proliferating Cells
- Autophagy has cytoprotective effects and other unique effects in dividing cells:
- Cytoprotective effects – see #10 above
- Reduced stem cell attrition
- Reduced ROS-induced cellular senescence
- Reduced oncogenic transformation
- Improved genetic stability
- Increased p62 degradation
- Anti-cancer effects via increased oncogene-induced senescence and oncogene-induced apoptosis
With aging, there is a decline in bone marrow stem cell function (hematopoeitic stem cells and mesenchymal stem cells) and stem cell number (MSCs only). Rapamycin restores the self-renewal capability of hematopoietic stem cells (HSCs). This improves the function of the immune system, of course assuming a lower dose of rapamycin than the immunosuppressive rapamycin dose given for preventing organ transplant rejection. Rapamycin can also reverse the stem cell loss that occurs in hair follicles and thereby prevent alopecia. mTOR accelerates cellular senescence by increasing the expression of p16/INK4a, p19/Arf, and p21/Cip1. These are all markers of cellular senescence and up regulating these tumor suppressors induces cellular senescence.
The tumor suppressor PTEN is just the opposite, however. Loss of the tumor suppressor PTEN induces a unique type of cellular senescence called “PTEN loss-induced cellular senescence” (PICS). PICS occurs with mTOR activation and can be reduced by inhibiting MDM2, which leads to an increase in p53 expression. This would inhibit autophagy. Rapamycin can preclude permanent (irreversible) cell-cycle arrrest due to inducible p21 expression. In this aspect, mTOR decreases proliferative potential and mediates stem cell attrition via senescence. Rapamycin can suppress this. This effect may be mediated by autophagy or by an autophagy-independent effect of mTOR inhibition.
More importantly, several oncogenes suppress autophagy. This includes Akt1, PI3K, Bcl-2 family anti-apoptotic proteins. Most of the proteins that stimulate autophagy also inhibit oncogenesis. This includes DAPK1, PTEN, TSC1, TSC2, LKB1/STK11, and Beclin-1. Autophagy can suppress oncogenesis through cell-autonomous effects described below:
- Improved quality control of mitochondria (less baseline ROS production)
- Enhanced genetic stability
- Removal of potentially oncogenic protein p62 via autophagy.
- Autophagy up regulation results in oncogene-induced senescence (via Ras)
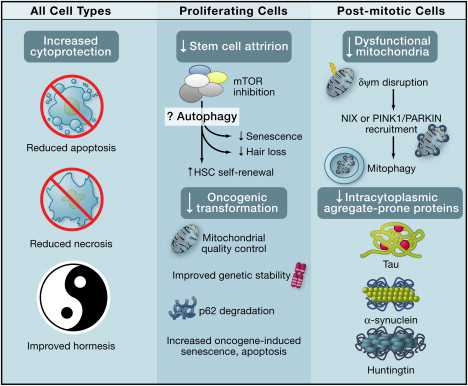
The diagram below shows the beneficial effects of autophagy on all cell types, specific benefits in proliferating cells, and specific benefits in post-mitotic cells.
Systemic Anti-Aging Effects of Autophagy Image source
12. Autophagy can reduce age-related dysfunction through systemic effects –
Autophagy also confers several beneficial anti-aging effects that are not due to cytoprotection, or other localized effects within the cell itself. This includes the following systemic benefits of autophagy:
- Defense against infections
- Innate immunity
- Inhibition of pro-inflammatory signaling
- Neuroendocrine effects of autophagy
Autophagy in dying antigen-presenting cells improves the presentation of the antigens to dendritic cells. In dendritic cells, autophagy improves antigen presentation to T cells. Autophagy in dying cells is also required for macrophage clearance of these dead/dying cells. This is how autophagy reduces inflammation. Autophagy helps keep ATP production going in these dying cells, providing energy for the key step in the lysophosphatidylcholine “find me” signaling as well as the phosphatidylserine ”flip flop” that is the “eat me” recognition signal for macrophage ingestion of the dying/dead cells. By helping macrophages find these cells and recognize that they are ready for macrophage ingestion, these cells do not rupture and spill their intracytoplasmic contents (this is what causes the inflammation with necrosis, where cell membrane rupture occurs).
When autophagy is working hand-in-hand with apoptosis, no inflammation occurs when a cell dies. This is a key beneficial role of autophagy in reducing inflammation. The decline in autophagy seen in aging may be in part the cause of age-induced type-2 diabetes. Here the peripheral tissues become insulin resistant. This may be due to the hepatic suppression of the Atg7 gene, which results in ER stress and insulin resistance. Induction of autophagy in specific neural populations may be sufficiency to reduce pathological aging.
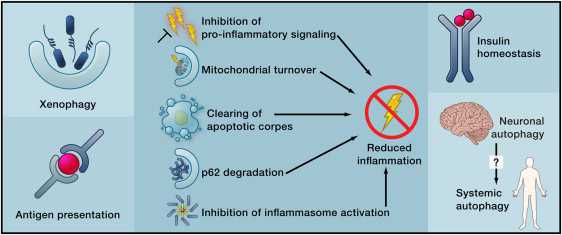
More effects of autophagy Image source
Beyond its cell-autonomous action, autophagy can reduce age-related dysfunctions through systemic effects. Autophagy may contribute to the clearance of intracellular pathogens and the function of antigen-presenting cells (left), reduce inflammation by several mechanisms (middle), or improve the function of neuroendocrine circuits (right).
13. Autophagy is necessary for maintaining the health of pools of adult stem cells
Frequent readers of this blog know that the writers believe that age-related decline of the health and differentiation capability of adult stem cells and increasing sensescence of those cells may be responsible for many of the effects we associate with aging. Thus, the positive roles of autophagy in keeping stem cells viable is of great interest to us.
See the comments under 11 above. Also, the June 2013 review publication Autophagy in stem cells provides “a comprehensive review of the current understanding of the mechanisms and regulation of autophagy in embryonic stem cells, several tissue stem cells (particularly hematopoietic stem cells), as well as a number of cancer stem cells.” Another such review is the June 2012 e-publication Tightrope act: autophagy in stem cell renewal, differentiation, proliferation, and aging.
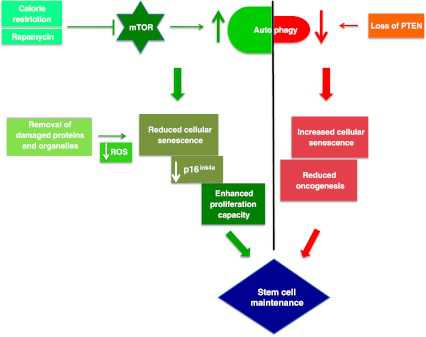
Image Source “Tightrope act inhibition of mTOR via caloric restriction (CR) or rapamycin induces autophagy. Autophagy clears away damaged proteins and organelles like defective mitochondria, thereby decreasing ROS levels and reducing genomic damage and cellular senescence, thus playing a crucial role in enhancing stem cell longevity. CR may also have a role in maintaining low levels of p16ink4a, a tumor suppressor protein, thus reducing the risk of cancer and promoting proliferation of stem cells. Oncogenesis is countered by loss of PTEN which elicits a p53-dependent prosenescence response to decrease tumorigenesis(ref)”
Only now are studies beginning to emerge that characterize the detailed roles of autophagy in maintaining stem cell health and differentiation viability. Autophagy in stem cells recapitulates the current state of understanding: “As a major intracellular degradation and recycling pathway, autophagy is crucial for maintaining cellular homeostasis as well as remodeling during normal development, and dysfunctions in autophagy have been associated with a variety of pathologies including cancer, inflammatory bowel disease and neurodegenerative disease. Stem cells are unique in their ability to self-renew and differentiate into various cells in the body, which are important in development, tissue renewal and a range of disease processes. Therefore, it is predicted that autophagy would be crucial for the quality control mechanisms and maintenance of cellular homeostasis in various stem cells given their relatively long life in the organisms. In contrast to the extensive body of knowledge available for somatic cells, the role of autophagy in the maintenance and function of stem cells is only beginning to be revealed as a result of recent studies. Here we provide a comprehensive review of the current understanding of the mechanisms and regulation of autophagy in embryonic stem cells, several tissue stem cells (particularly hematopoietic stem cells), as well as a number of cancer stem cells. We discuss how recent studies of different knockout mice models have defined the roles of various autophagy genes and related pathways in the regulation of the maintenance, expansion and differentiation of various stem cells. We also highlight the many unanswered questions that will help to drive further research at the intersection of autophagy and stem cell biology in the near future.”
Another very-recent finding related to autophagy and stem cells is reported in the March 31, 2013 paper FIP200 is required for maintenance and differentiation of postnatal neural stem cells. “These data reveal that FIP200-mediated autophagy contributes to the maintenance and functions of NSCs through regulation of oxidative state.” FIP200 is “a gene essential for autophagy induction in mammalian cells.”
Exercising control over autophagy may prove useful for efficiently generating induced pluripotent stem cells. According to the 2012 publication Autophagy in stem cell maintenance and differentiation: “We also discuss a possible role for autophagy during cellular reprogramming and induced pluripotent stem (iPS) cell generation by taking advantage of ATP generation for chromatin remodeling enzyme activity and mitophagy. Finally, the significance of autophagy modulation is discussed in terms of augmenting efficiency of iPS cell generation and differentiation processes.”
A steady stream of research continues to reveal new insights on the roles that autophagy plays in stem cells. For example, the April 2013 publication FOXO3A directs a protective autophagy program in haematopoietic stem cells reports: “Here we identify autophagy as an essential mechanism protecting HSCs from metabolic stress. We show that mouse HSCs, in contrast to their short-lived myeloid progeny, robustly induce autophagy after ex vivo cytokine withdrawal and in vivo calorie restriction. We demonstrate that FOXO3A is critical to maintain a gene expression program that poises HSCs for rapid induction of autophagy upon starvation. Notably, we find that old HSCs retain an intact FOXO3A-driven pro-autophagy gene program, and that ongoing autophagy is needed to mitigate an energy crisis and allow their survival. Our results demonstrate that autophagy is essential for the life-long maintenance of the HSC compartment and for supporting an old, failing blood system.”
14. Autophagy is a key step in activating the Nrf2 pathway. And Nrf2 expression can in turn regulate autophagy.
The importance of the Nrf2 stress-response pathway and its role in generating health has been one of the frequent topics of discussion in this blog. See specifically the blog entries The pivotal role of Nrf2. Part 1, Part 2, Part 3, and Nrf2 and cancer chemoprevention by phytochemicals. We know now that autophagy plays a key role in Nrf2 activation, via p62-dependent autophagic degradation of Keap1. See, for example, the 2012 publication Sestrins Activate Nrf2 by Promoting p62-Dependent Autophagic Degradation of Keap1 and Prevent Oxidative Liver Damage. We also know that, in turn, Nrf2 expression can regulate autophagy. See for example the March 2013 publication Regulation of Cigarette Smoke (CS)-Induced Autophagy by Nrf2.
15. Autophagy and aging
We are starting to understand why autophagy stops working well when a person grows old - why autophagy does not work as well as you age. Among the reasons are:
a. Failure to form autophagosomes - with aging, there appears to be a failure for autophagosomes to form, possibly due to macroautophagy enhancers (glucagon).
b. Failure of fusion - with aging, there appears to be a failure of lysosomes to fuse with autophagosomes.
c. Negative signaling from insulin or insulin receptors - with aging, insulin signaling or insulin receptor signaling activates mTOR in cells.
d. Mitophagy does not work as well in aging.
e. Autophagy decline probably also results in energy (ATP production) decline.
16. Practical interventions to promote autophagy
There are a number of practical ways to promote autophagy. Specifically, in partial recap of the above:
- Fasting activates Autophagy - caloric restriction affects 5 molecular pathways that activate autophagy
- Sunlight, Vitamin D and Klotho activate Autophagy - there are three ways through which UV light, Vitamin D, and the Klotho pathway activate autophagy via inhibiting the insulin/IGF-1 pathway
- Rapamycin activates Autophagy - there are two ways through which mTOR inhibitors activate autophagy – TORC1 and TORC2 mechanisms
- Caffeine activates Autophagy - Caffeine can activate autophagy via an mTOR-dependent mechanism
- Green tea activates Autophagy - ECGC can activate autophagy via an mTOR-dependent mechanism
- Metformin activates Autophagy - metformin can activate autophagy via AMPK activation – mTOR-dependent and mTOR-independent mechanisms
- Lithium activates Autophagy - lithium and other compounds can activate autophagy by inhibiting inositol monophosphate and lower IP3 levels – an mTOR-independent mechanism
- Resveratrol activates Autophagy – there are four 4 ways through which resveratrol can activate autophagy – via mTOR-dependent and mTOR-independent mechanisms
- Spermidine activates Autophagy - how spermidine activates autophagy via histone protein deacetylation – mTOR-indepdendent mechanism
- Hypoxia activates Autophagy - intermittent hypoxia can increase autophagy via HIF-1a
- Phytosubstances which activate the Nrf2 pathway can activate Autophagy. These are many and include soy products and hot chili peppers.
In addition, these lesser-known substances can also activate autophagy:
Amiodarone low dose Cytoplasm – midstream yes Calcium channel blocker => TORC1 inhibition. Also, a mTOR-independent autophagy inducer
- Fluspirilene low dose Cytoplasm – midstream yes Dopamine antagnoists => mTOR-dependent autophagy induction
- Penitrem A low dose Cytoplasm – midstream yes high conductance Ca++activated K+ channel inhibitor => mTOR-dependent autophagy inducer
- Perihexilene low dose Cytoplasm- midstream yes 1. TORC1 inhibition
- Niclosamide low dose Cytoplasm- midstream yes 1. TORC1 inhibition
- Trehalose 100 mM Cytoplasm – midstream supplement 1. activates autophagy via an mTOR-independent mechanism
- Torin-1 low dose Cytoplasm – midstream no 1. mTOR inhibition (much more potent than rapamycin)
- Trifluoperazine low dose Cytoplasm – midstream yes Dopamine antagonists => mTOR-dependent autophagy induction
Wrapping it up
Here are some of the main points above covered:
- Autophagy is like having a Pac man inside each of your cells, chasing down, eating up and recycling dysfunctional organelles, proteins and protein aggregates. It has three forms: i. chaperone-mediated autophagy, ii. microautophagy and iii. macroautophagy. The last is the most important one.
- Autophagy is a stress response and behaves according to the principles of hormesis.
- Autophagy can retire and eat up old mitochondria which have become electron-leaking engines.
- Autophagy solves the problem of high baseline levels of reactive oxygen and nitrogen species.
- Autophagy does not require proteins to be unfolded for it to work and therefore can perform housekeeping tasks undoable by the other cell-level house cleaning system, the ubiquitin-proteasome system.
- Autophagy gets rid of the protein aggregates that can make you loose your memory or walk slow as you grow old – those associated with Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, ALS, CTE, and other neurodegenerative conditions.
- Autophagy keeps adult stem cells healthy and facilitates their capability to differentiate to make normal somatic body cells.
- Autophagy prevents inflammation - it works hand-in-hand with apoptosis to help the body get rid of dying cells without inducing cell rupture and inflammation.
- Autophagy prevents cancer - it helps maintain genetic stability, prevents epigenetic gene silencing. And it helps promote oncogene-induced cellular senescence for cancer prevention.
- Autophagy saves the lives of cells by preventing unnecessary cellular apoptosis and cell necrosis.
- Autophagy is involved in Nrf2 activation and to some extent Nrf2 expression negatively regulates autophagy.
- Autophagy keeps your bone marrow stem cell population alive and functional.
- Autophagy helps with infections – it helps clear intracellular pathogens such as bacteria and viruses.
- Autophagy improves the innate immune response.
- We are starting to understand why autophagy declines with aging.
- While autophagy declines with aging, it can exercise multiple effects to slow aging down. It inhibits the major mechanisms of aging such as cellular senescence, protein aggregate build-up, stem cell loss, epigenetic gene silencing, telomere shortening, and oxidative damage to proteins, lipids, and DNA.
- There are many practical ways to activate Autophagy like consuming green tea and caffeine, and some less-practical ones.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言