FDA批准**植入式听力器械用于特定听力丧失的成年患者
2014-03-28 fyc5078 DXY
3月20日,美国FDA批准首款植入式器械用于双耳严重或深度感音神经高频率声音听力丧失,但借助(或不用)助听器仍能听到低频率声音的18岁及以上患者。这款名为Nucleus Hybrid L24 Cochlear Implant System的器械可能会帮助到患者。 感音神经听力丧失是最常见形式的听力丧失,在内耳(耳蜗)出现损伤时会发生。它可能由老龄化、遗传、暴露于噪声中、对内耳
3月20日,美国FDA批准首款植入式器械用于双耳严重或深度感音神经高频率声音听力丧失,但借助(或不用)助听器仍能听到低频率声音的18岁及以上患者。这款名为Nucleus Hybrid L24 Cochlear Implant System的器械可能会帮助到患者。
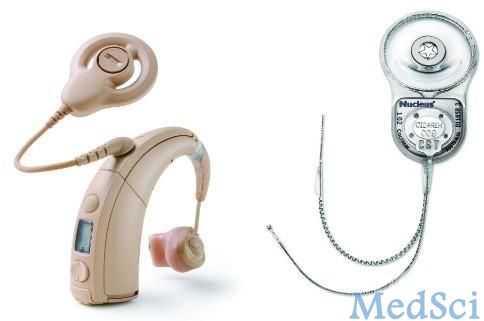
感音神经听力丧失是最常见形式的听力丧失,在内耳(耳蜗)出现损伤时会发生。它可能由老龄化、遗传、暴露于噪声中、对内耳有毒性的药物(如抗生素)及某些其它疾病引起。患有严重或深度感音神经高频率声音听力丧失的人可能对听到微弱的声音、理解声调较高的人、听到某些语音,及在某些情况下听到高声调的汽车鸣笛声或通常的安全警报(如烟雾报警器)有难度。
Nucleus Hybrid L24 Cochlear Implant System将耳蜗植入与助听器功能结合在一起。这款电子器械由外部传声器和语音处理器构成,语音处理器获得来自环境的声音,然后将其转换成电脉冲。脉冲通过一小束植入电极传输到耳蜗,产生听觉,使用者要学会将这种听觉与他们记忆的中频及高频声音联系起来。器械的助听器部分被插入外耳道,像一个传统的助听器,它可以在低频率范围内放大声音。
FDA评价了一项临床研究,受试者由50名患有严重至深度高频听力丧失但仍有明显低频率听力的患者组成。患者在该器械被植入前或植入后接受了检测。在器械激活后的6个月内,与患者植入器械前使用常规助听器械时的基线值相比,大多数患者报告对单词及句子的识别有统计学意义的明显改善。这款器械也进行了非临床测试,包括该器械的电器元件、生物相容性和持久性等。
在参与研究的50名受试者中,68%的人经历了一些预料之内的不良事件,如低频率听力丧失,耳鸣、电极故障及头晕。22名受试者其植入耳朵发展成深度或完全低频率听力丧失,其中6名受试者经历了额外的手术,以用标准的耳蜗植入替代Nucleus Hybrid L24 Cochlear Implant System。
虽然低频率听力丧失风险令人担忧,但FDA认为对那些不能受益于传统助听器的患者来说,该器械的整体收益超过其风险。Nucleus Hybrid L24 Cochlear Implant System由总部位于澳大利亚新南威尔斯州的 Cochlear 公司生产。
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言











#FDA批准#
25
#植入式#
27
#听力#
33
#植入#
30