强生丙肝新药OLYSIO(simeprevir)获FDA批准
2013-11-25 tomato 生物谷
强生(JNJ)11月22日宣布,丙肝新药OLYSIO(simeprevir)已获FDA批准,联合聚乙二醇干扰素和利巴韦林(ribavirin),用于基因型1慢性丙型肝炎成人患者代偿性肝脏疾病(包括肝硬化)的治疗。 此前,FDA已于今年5月授予simeprevir新药申请(NDA)优先审查资格,同时该药于今年10月获得了FDA顾问委员会建议批准的积极意见。 Simeprevir监管文件的提交
强生(JNJ)11月22日宣布,丙肝新药OLYSIO(simeprevir)已获FDA批准,联合聚乙二醇干扰素和利巴韦林(ribavirin),用于基因型1慢性丙型肝炎成人患者代偿性肝脏疾病(包括肝硬化)的治疗。
此前,FDA已于今年5月授予simeprevir新药申请(NDA)优先审查资格,同时该药于今年10月获得了FDA顾问委员会建议批准的积极意见。
Simeprevir监管文件的提交,部分由3个关键性III期临床试验数据支持:QUEST-1和QUEST-2在初治患者中开展,PROMISE则在基于干扰素疗法治疗后复发的患者中开展。
丙型肝炎(HCV)是一种血源性传染性肝脏疾病,若不及时治疗,可能对肝脏造成重大损害。在美国,约有320万丙型肝炎患者,每年约有1.5万人死于该病,大多死于丙型肝炎相关疾病,如肝硬化和肝癌。
关于OLYSIO(simeprevir):
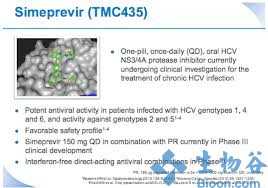
Simeprevir是新一代NS3/4A蛋白酶抑制剂,为每日一次的口服药物,由Medivir公司和杨森(Janssen)联合开发,用于治疗慢性丙型肝炎成年患者的代偿性肝病,包括各个阶段的肝纤维化,其工作原理是通过阻断蛋白酶,来抑制HCV在肝脏细胞中的复制。
今年9月,simeprevir(在日本的商品名为Sovriad)获日本劳动卫生福利部(MHLW)批准,与聚乙二醇化干扰素和利巴韦林(ribavirin)联合用药,用于基因型-1慢性丙型肝炎病毒(HCV)感染者的治疗,这是simeprevir获得的全球首个监管批准。
simeprevir是一种新的直接作用抗病毒药物,也是第二代蛋白酶抑制剂,给药方式为:simeprevir+聚乙二醇干扰素+利巴韦林联合治疗12周,随后进行聚乙二醇干扰素+利巴韦林治疗12周或36周。
英文原文:OLYSIO? (simeprevir) Receives FDA Approval for Combination Treatment of Chronic Hepatitis C
OLYSIO? is the first once-daily protease inhibitor approved for the treatment of chronic hepatitis C in a combination antiviral regimen for adults with compensated liver disease
TITUSVILLE, N.J. (November 22, 2013) – Janssen Therapeutics, Division of Janssen Products, LP (Janssen), announced today the U.S. Food and Drug Administration (FDA) has approved OLYSIO? (simeprevir), an NS3/4A protease inhibitor, for the treatment of chronic hepatitis C infection as part of an antiviral treatment regimen in combination with pegylated interferon and ribavirin in genotype 1 infected adults with compensated liver disease, including cirrhosis. OLYSIOTM may benefit patients with chronic hepatitis C, including those who are treatment na?ve or who have failed prior interferon-based therapy.
Chronic hepatitis C is a blood-borne infectious disease of the liver that affects approximately 3.2 million people in the United States.
OLYSIO? works by blocking the viral protease enzyme that enables the hepatitis C virus (HCV) to replicate in host cells. The goal of treatment for chronic hepatitis C is cure, also known as sustained virologic response (SVR), which is defined as undetectable levels of HCV in the patients’ blood 12 to 24 weeks after the end of treatment. For treatment-na?ve and prior-relapser patients, a fixed treatment regimen of 12 weeks of OLYSIO? combined with 24 weeks of pegylated interferon and ribavirin is recommended. For prior partial- and null-responder patients, a treatment regimen of 12 weeks of OLYSIO? combined with 48 weeks of pegylated interferon and ribavirin is recommended.
“Given the complexity of the condition, OLYSIO? was studied in a number of different patient populations, including individuals who have relapsed or failed to respond to previous treatments,” said Douglas Dieterich, M.D., Professor of Medicine in the Division of Liver Diseases, Mount Sinai School of Medicine, and OLYSIO? clinical trial investigator. “The FDA approval of OLYSIO? is an important milestone for people living with chronic hepatitis C as it means that patients have a new treatment option with the potential to cure this challenging disease.”
OLYSIO? is a prescription medicine used with other antiviral medicines, pegylated interferon and ribavirin, to treat genotype 1 chronic hepatitis C in adults with stable liver problems. OLYSIO? must not be taken alone. The efficacy of OLYSIO? in combination with peginterferon and ribavirin is greatly decreased in patients who have genotype 1a Q80K. Please talk to your doctor about testing for genotype 1a Q80K and using a different therapy when genotype 1a Q80K is present. It is not known if OLYSIO? is safe and effective in children under 18 years of age.
The New Drug Application (NDA) filed by Janssen Research & Development, LLC, for OLYSIO? was based in part on efficacy and safety results from three pivotal Phase 3 studies – QUEST-1 and QUEST-2 in treatment-na?ve patients and PROMISE in patients who have relapsed after prior interferon-based treatment – as well as data from the Phase 2b ASPIRE study in prior non-responder patients. Each of the studies evaluated OLYSIO? dosed once daily in combination with pegylated interferon and ribavirin versus treatment with placebo plus pegylated interferon and ribavirin.
Results from a pooled analysis of QUEST-1 and QUEST-2 demonstrated that 80 percent of treatment-na?ve patients in the group receiving OLYSIO? achieved sustained virologic response 12 weeks after the end of treatment (SVR12), compared with 50 percent of patients in the placebo groups. In PROMISE, 79 percent of prior-relapser patients in the simeprevir group of the study achieved SVR12 compared with 37 percent of patients in the placebo group. Results from ASPIRE demonstrated that use of OLYSIO? led to sustained virologic response 24 weeks after the end of treatment (SVR24) in 65 percent of prior partial-responder patients and 53 percent of prior-null responder patients compared with 9 percent and 19 percent of prior partial- and null-responder patients in the placebo groups, respectively.
In the QUEST-1 and QUEST-2 studies, among genotype 1a treatment-na?ve patients receiving OLYSIO? who had the Q80K polymorphism (a naturally occurring variation in the HCV NS3/4A protease enzyme), 58 percent achieved SVR12 versus 84 percent of patients without the Q80K polymorphism. In the placebo arm, 52 percent of patients with the Q80K polymorphism achieved SVR12. In the PROMISE study, among prior-relapser patients with the Q80K polymorphism who received OLYSIO?, 47 percent achieved SVR12 versus 78 percent of patients without the polymorphism. In the placebo arm, 30 percent of patients with the Q80K polymorphism achieved SVR12.
“As an advocate working with the hepatitis C community, I’m pleased to know that Janssen has been working to make sure OLYSIO? will be reasonably priced and available to the patients who need it,” said Sue Simon, President of the Hepatitis C Association. “It is notable that in addition to introducing a new treatment option for patients, Janssen is establishing comprehensive programs to support and assist patients in their treatment journey.
Janssen has launched OLYSIOTM Support, a comprehensive support program designed in partnership with the HCV community to assist in the hepatitis C treatment journey so that patients and caregivers – and their healthcare providers – can focus on treatment. To register for OLYSIOTM Support or for additional information, please visit OLYSIO.com.
About OLYSIO? (simeprevir)
OLYSIO? (simeprevir) is an NS3/4A protease inhibitor jointly developed by Janssen R&D Ireland and Medivir AB and indicated in the U.S. for the treatment chronic hepatitis C infection in combination with pegylated interferon and ribavirin in HCV genotype 1 infected subjects with compensated liver disease, including cirrhosis.
Janssen is responsible for the global clinical development of OLYSIO? and has exclusive, worldwide marketing rights, except in the Nordic countries. Medivir AB will retain marketing rights for OLYSIO? in these countries under the marketing authorization held by Janssen-Cilag International NV. The treatment was approved in September 2013 in Japan under the trade name SOVRIAD? and in November 2013 in Canada under the trade name GALEXOS? for the treatment of genotype 1 hepatitis C. A Marketing Authorisation Application was submitted to the European Medicines Agency (EMA) in April 2013 by Janssen-Cilag International NV seeking approval of OLYSIO? for the treatment of genotype 1 or genotype 4 chronic hepatitis C. To date, more than 3,700 patients have been treated with OLYSIO? in clinical trials.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言












#丙肝新药#
24
#FDA批准#
34
#Simeprevir#
23
#强生#
27