NEJM:开启医学新时代:体内CRISPR基因编辑临床试验结果公布,安全有效
2021-06-28 “E药世界”公众号 “E药世界”公众号
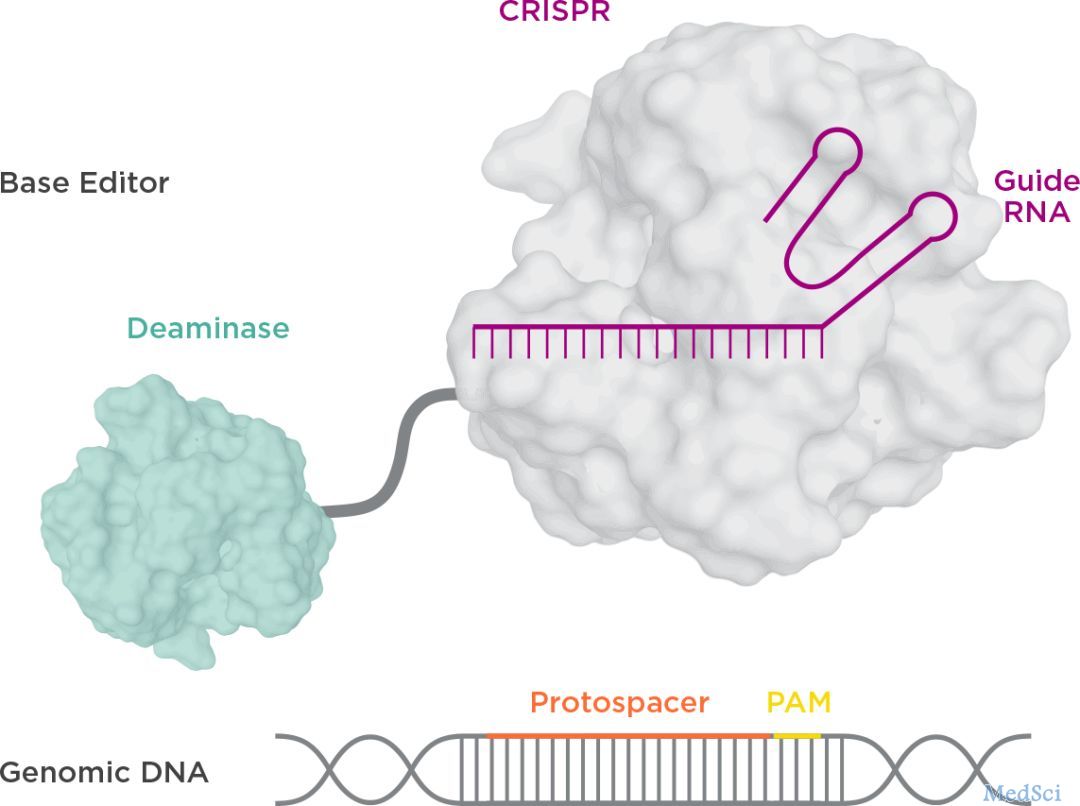
2012年8月17日,詹妮弗·杜德娜(Jennifer Doudna)和埃玛纽埃尔·卡彭蒂耶(Emmanulle Charpentier)合作,在 Science 杂志发表了基因编辑史上的里程碑论文,
2012年8月17日,詹妮弗·杜德娜(Jennifer Doudna)和埃玛纽埃尔·卡彭蒂耶(Emmanulle Charpentier)合作,在 Science 杂志发表了基因编辑史上的里程碑论文,成功解析了CRISPR/Cas9基因编辑的工作原理。她们因这项成就荣获2020年诺贝尔化学奖。

以CRISPR/Cas9为代表的基因编辑技术,大大加速了基因治疗的快速发展,为许多原本无药可医的遗传疾病带来了巨大的希望。
2021年6月26日,国际顶尖医学期刊《新英格兰医学期刊》NEJM 发表了题为:CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis 的临床实验论文。
诺奖得主詹妮弗·杜德娜(Jennifer Doudna)创立的 Intellia Therapeutics 和再生元合作开发的治疗转甲状腺素蛋白淀粉样变性(ATTR)的CRISPR基因编辑疗法NTLA-2001,在6名患者的临床试验中安全有效。
据悉,这也是首个公布的体内CRISPR基因编辑疗法的临床试验结果。
转甲状腺素蛋白淀粉样变性(ATTR),是一种危及生命的严重罕见遗传疾病,据估计,全球大约有5万名患者,该疾病的特征是错误折叠的转甲状腺素蛋白(TTR)蛋白在组织中逐渐积累,主要是神经和心脏。
该研究使用的 NTLA-2001 疗法,是一种体内基因编辑治疗,通过脂质纳米颗粒(LNP)递送载体,将携带靶向致病基因TTR基因的sgRNA和优化的spCas9蛋白的mRNA序列,递送至肝脏。
临床数据显示,在这6名接受治疗的患者中,3名患者接受0.1mg/kg剂量,3例患者接受0.3mg/kg剂量。接受治疗28天后,两种不同剂量的患者血浆种TTR蛋白水平分别平均下降52%和87%。且未观察到严重不良反应。
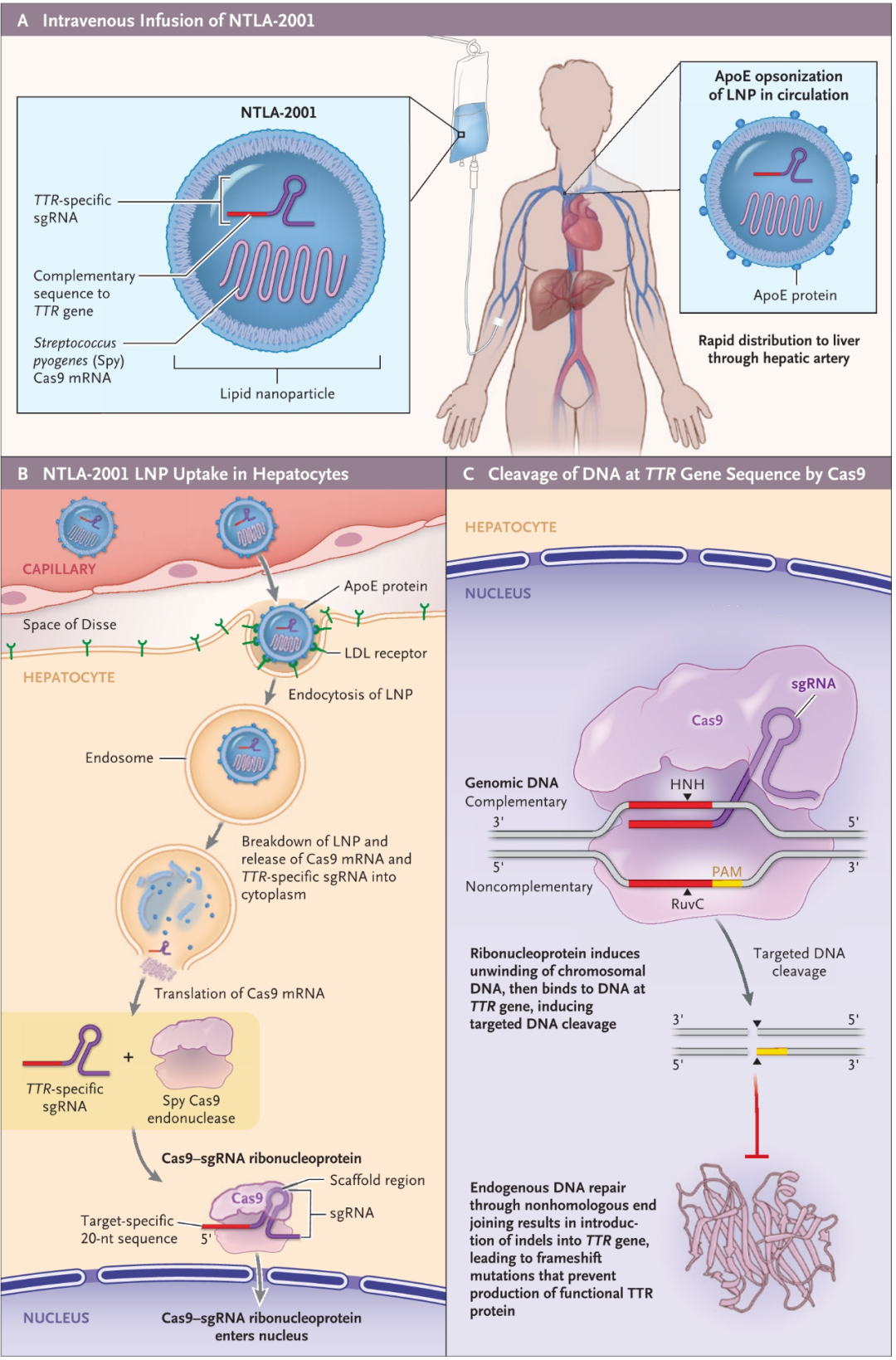
这是有史以来第一个临床数据,证明可以精确地在体内进行CRISPR基因编辑,通过单次静脉输注CRISPR系统来治疗遗传疾病。
Intellia Therapeutics 的 CEO John Leonard 表示,这项临床试验结果表明,NTLA-2001 可能通过单次注射阻止转甲状腺素蛋白淀粉样变性(ATTR)疾病的发展以及逆转其破坏性并发症。该临床试验还解决了CRISPR向肝脏递送的难题,为其他遗传疾病的治疗打开了大门,他还表示,将迅速推进该疗法,同时扩大现有研发管线。
Intellia 现在的研发管线
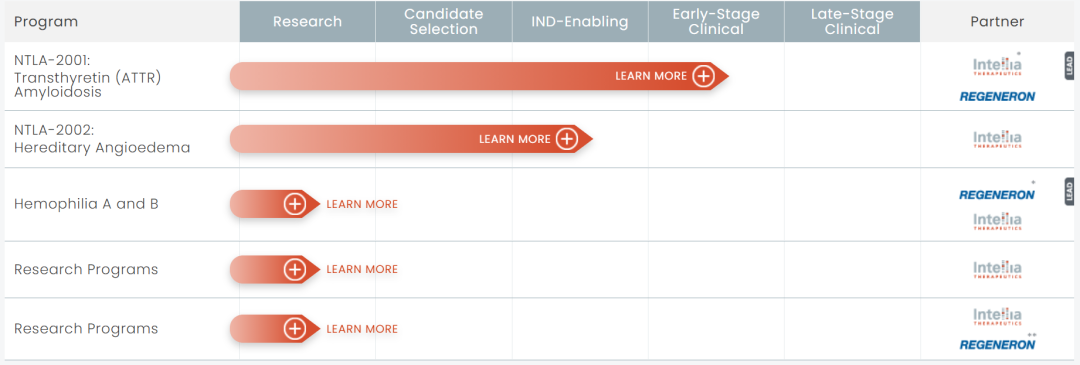
体内基因编辑疗法
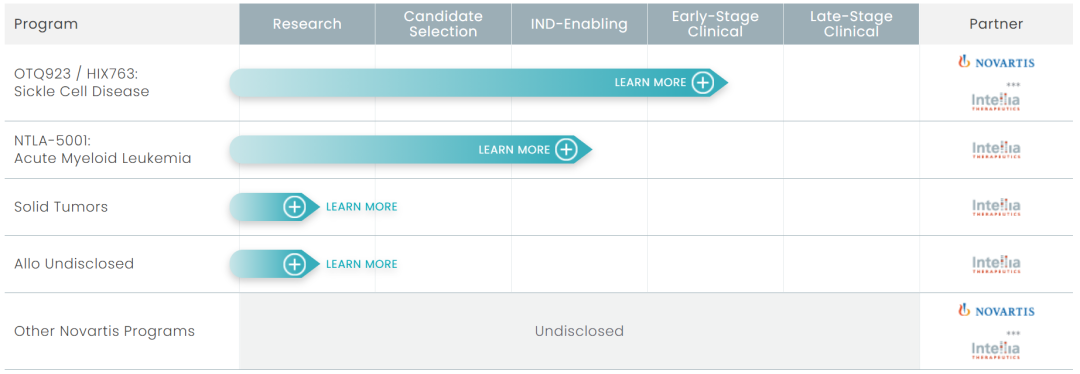
体外基因编辑疗法
实际上,首个开展体内CRISPR基因治疗的是张锋创立的 Editas Medicine,2020年3月4日,艾尔建和 Editas Medicine 联合宣布:CRISPR疗法AGN-151587(EDIT-101)治疗先天性黑蒙症10型(LCA10)的I/II期临床试验,已完成首例患者给药。
该临床试验使用的是腺相关病毒(AAV)将CRISPR基因编辑系统通过注射递送到眼睛。这是全球首个体内CRISPR基因编辑临床试验,也是全球首例CRISPR基因编辑的在体给药,据悉,该临床试验结果将于今年9月份公布。

而在2020年12月5日,《新英格兰医学期刊》NEJM 发表了 CRISPR Therapeutics 和 Vertex Pharmaceuticals 联合开发的利用CRISPR/Cas9基因编辑技术治疗β-地中海贫血症和镰状细胞病的临床试验。
证实了体外CRISPR基因编辑技术在治疗这两种遗传疾病上的安全性和有效性。

这项体内CRISPR基因编辑疗法临床试验结果,大大扩展了CRISPR基因编辑疗法的应用范围,能够直接通过注射在体内进行高效基因编辑,为许多遗传疾病的治疗开辟了新的途径,可以说是开启了医学新时代。
原始出处:
Julian D. Gillmore, et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. NEJM, June 26, 2021. DOI: 10.1056/NEJMoa2107454
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言










#CRISPR基因编辑#
55
#结果公布#
49
#CRISPR#
32
#新时代#
43
顶刊就是顶刊,谢谢梅斯带来这么高水平的研究报道,我们科里同事经常看梅斯,分享梅斯上的信息
52