【综述】| P53与肿瘤代谢的最新研究进展
2024-01-15 中国癌症杂志 中国癌症杂志 发表于陕西省
本文就P53与葡萄糖、脂肪酸、氨基酸和核苷酸代谢的关系进行综述,并梳理肿瘤发展中P53在这些代谢调控中的复杂机制和最新研究进展。
[摘要] 由TP53编码的P53蛋白是防止细胞癌变所需的关键因子,有广泛而强大的功能。P53在诱导细胞周期停滞、DNA损伤修复、细胞凋亡和衰老等过程中发挥重要作用,且这些功能的丧失不会使P53失去抑癌活性。新陈代谢是生命的基础,代谢异常导致多种疾病如肿瘤的发生,是癌症进展的主要驱动力之一。最近研究发现P53在调节全身代谢中起关键作用。P53介导的细胞代谢调节是控制肿瘤发生、发展的基本机制,有助于抑制肿瘤活性。本文就P53与葡萄糖、脂肪酸、氨基酸和核苷酸代谢的关系进行综述,并梳理肿瘤发展中P53在这些代谢调控中的复杂机制和最新研究进展。
[关键词] P53;糖代谢;脂肪酸氧化;核苷酸代谢;氨基酸代谢
[Abstract] P53 is a key factor encoded by the TP53, and it prevents cells from becoming cancerous and has a wide range of powerful functions. p53 is found to play an important role in inducing DNA repair, apoptosis, cell cycle arrest and senescence, and the loss of these functions does not abrogate P53’s tumor suppressive activity. Metabolism is the basis of life, and metabolic abnormalities can lead to a variety of diseases, including tumors, and is one of the main drivers of cancer progression. It has recently been discovered that P53 plays a key role in regulating metabolism. P53-mediated regulation of cell metabolism is a fundamental mechanism controlling cancer occurrence and development and contributes to its tumor suppressive activity. Here, this article reviewed the relationship between P53 and glucose, fatty acid, amino acid and nucleotide metabolism, and discussed the complex mechanism and the latest research progress of P53 in the metabolic regulation in tumor development.
[Key words] P53; Glucose metabolism; β-oxidation; Nucleotide metabolism; Amino acid metabolism
p53是人类肿瘤中常见的抑癌基因之一,是分子生物学和肿瘤学领域的“明星分子”。p53主要通过调控下游靶基因来发挥作用,在细胞核中起转录因子的作用[1],可对内外源性损伤如癌基因激活、缺氧、DNA损伤、热和冷休克、蛋白质错误折叠及端粒缩短做出反应。作为应对细胞应激的关键组分,P53可被活化的癌基因、贫乏的营养物质等应激信号激活[2];P53在正常细胞的细胞核内与E3泛素连接酶MDM2和MDMX相互作用[3],被泛素化后转运到细胞质中由蛋白酶体降解,使P53维持低水平。P53长期被关注主要因为其通过控制细胞衰老、凋亡和DNA修复来抑制肿瘤的巨大潜力。P53通过多种途径使细胞适应应激,并维持代谢稳态,在细胞新陈代谢中发挥关键作用。因此,除了p53在癌症中的功能及被看作是“基因组的守护者”外,也被认为是“代谢稳态的守护者”。
1 P53与糖代谢
葡萄糖是人体生命活动所需的重要营养物质,为人体提供能源和碳源,一旦被细胞摄取,先在细胞质中转化为丙酮酸,而后在有氧环境下于正常细胞的线粒体内彻底分解产生大量腺苷三磷酸(adenosine triphosphate,ATP)。葡萄糖转运蛋白(glucose transporters,GLUT)促进细胞摄取葡萄糖,而P53能间接调节Ras相关关联糖尿病因子(Ras-related associated with diabetes,RRAD)的转录来抑制GLUT1易位到质膜[4],或限制对氧磷酶2(paraoxonase 2,PON2)和核因子-κB(nuclear factor Kappa-B,NF-κB)的激活分别下调GLUT1和GLUT3[5-6];也可直接抑制GLUT4和GLUT12的表达来减少葡萄糖跨膜转运[6-7](图1)。此外,P53还可以抑制肝脏、骨骼肌和脂肪组织对胰岛素介导的葡萄糖摄取[8]。上述证据表明P53在糖代谢中发挥重要作用。
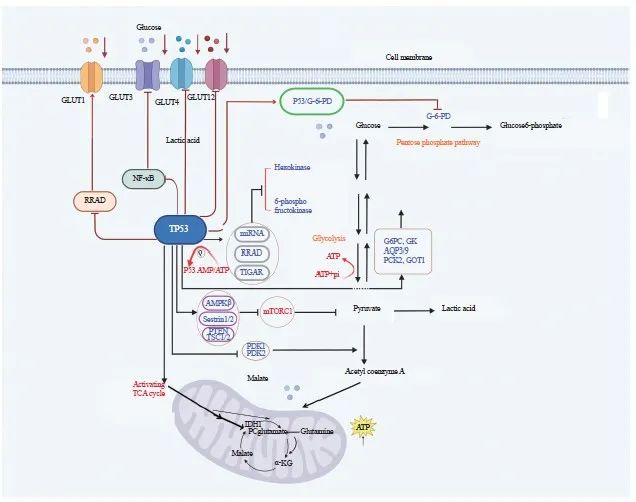
图1 P53调节糖代谢
Fig. 1 P53 regulates the glucose metabolism
The P53 regulates the transcription of RRAD to inhibit GLUT1 translocation to the plasma membrane or limit the activation of PON2 and NF-κB to downregulate GLUT1 and GLUT3, respectively; P53 can also directly inhibit GLUT 4 and GLUT12 expression to reduce glucose transmembrane transport. Moreover, P53 inhibits glycolysis and thus cancer cell growth through various ways. TIGAR: TP53-inducible glycolysis and apoptosis regulator; ADP: Adenosine diphosphate; G6PC: Glucose-6-phosphatase catalytic; GK: Glycerokinase; AQP3/9: Aquaporin 3/9; PCK2: Phosphoenolpyruvate carboxykinase 2; PDK: Pyruvate dehydrogenase kinase; AMPKβ: AMP-activated protein kinase β; Sestrin1/2: Antioxidant genes 1/2; PTEN: Phosphatase and tensin homolog deleted on chromosome ten; TSC1/2: Tuberous sclerosis complex 1/2; IDH1: Isocitrate dehydrogenase 1; TCG cycle: Tricarboxylic acid cycle; α-KG: α-Ketoglutarate; G-6-PD: Glucose-6-phosphate dehydrogenase; P53/G-6-PD: Combination of glucose-6-phosphate dehydrogenase and P53.
1.1 P53与糖酵解、糖异生
糖酵解异常是癌细胞最具特征性的代谢变化,癌细胞倾向于糖酵解而非氧化磷酸化(oxidative phosphorylation,OXPHOS)来产生中间代谢物和能量,即Warburg效应[9], Warburg效应满足了癌细胞对营养的巨大需求[10]。P53通过多种方式抑制糖酵解从而抑制癌细胞生长:P53调控miRNA和RRAD抑制葡萄糖-6-磷酸异构酶(glucose-6-phosphate isomerase,G6PI)、β-烯醇化酶(β-enolase)、己糖激酶1/2(hexokinase 1/2,HK1/2)和磷酸葡萄糖变位酶(Phosphoglucomutase,PGM)等几种参与糖酵解的酶[11-12]从而抑制糖酵解,如P53降低HK2的mRNA稳定性来抑制葡萄糖转化为葡萄糖-6-磷酸(glucose-6-phosphate,G6P)[13]。P53通过抑制6-磷酸果糖激酶(6-phosphofructo kinase,PFK)的转录或激活TP53诱导的糖酵解和细胞凋亡调节因子(TP53-inducible glycolysis and apoptosis regulator,TIGAR)和溶质载体16家族成员1(solute carrier family 16 member 1,SLC16A1)的转录来抑制糖酵解[4,14]。此外,活化的哺乳动物雷帕霉素复合物1(mammalian target of rapamycin complex 1,mTORC1)促进糖酵解,P53通过诱导AMP活化蛋白激酶β(AMP-activated protein kinase β,AMPKβ)、抗氧化基因1/2(antioxidant genes 1/2,Sestrin1/2)、人第10号染色体上缺失的磷酸酶及张力蛋白同源物基因(phosphatase and tensin homolog deleted on chromosome ten,PTEN)和结节性硬化症复合体1/2(tuberous sclerosis complex 1/2,TSC1/2)等若干负调节mTORC1活性的基因,来抑制糖酵解[15-16](图1)。已知低氧诱导因子1α(hypoxia inducible factor-1α,HIF-1α)激活乳酸脱氢酶A (lactate dehydrogenase A,LDHA),丙酮酸在LDHA/B的作用下为三羧酸(tricarboxylic acid,TCA)循环提供燃料,促进糖酵解[17],而由P53诱导帕金森氏病蛋白2(Parkinson disease protein 2,PARK2)编码的E3泛素连接酶Parkin能泛素化和降解HIF-1α,从而抑制糖酵解[18]。最近研究发现,若细胞摄取葡萄糖被抑制,机体AMP/ATP比值增加,能激活AMPK[19]而磷酸化并激活P53[7],形成P53与葡萄糖代谢间的反馈回路,使糖酵解向OXPHOS转变[20]。Cullin RING E3连接酶(Cullin RING E3 ligase,CRL)与其特异Den多克隆抗体CSN保持高度亲和作用,葡萄糖可诱导CSN-CRL4解离,并组装成P53 E3连接酶CRL4COP1,该酶以P53为靶点介导糖诱导的P53降解,促进糖酵解和癌细胞增殖,通过抑制CRL4COP1-P53相互作用可阻止P53降解,继而抑制糖酵解[21]。
糖异生即细胞合成葡萄糖,P53抑制糖酵解有助于糖异生的进行。Liu等[22]研究发现,P53通过诱导甘油激酶(glycerokinase,GK)、磷酸烯醇式丙酮酸羧激酶2(phosphoenolpyruvate carboxykinase 2,PCK2)、葡萄糖-6-磷酸酶催化亚基(glucose-6-phosphatase catalytic,G6PC)、水通道蛋白3/9(aquaporin 3,AQP3/9)和谷草转氨酶1(glutamic-oxaloacetic transaminase 1,GOT1)的表达,增强肝脏产生葡萄糖[23](图1)。P53还能激活泛酸激酶1(pantothenate kinase 1,PANK1)以增加细胞内乙酰辅酶A的水平并促进糖异生[24]。然而,也有研究[25]显示,P53通过激活沉默调节蛋白6(sirtuin 6,SIRT6)来抑制G6PC和PCK1,进而抑制糖异生。对于这些争议,或许是由于不同的饲养条件、特定的遗传背景或不同的微生物群影响了P53对关键代谢活动的控制[2,25-26]。
1.2 P53与TCA循环、磷酸戊糖途径(pentose phosphate pathway,PPP)
TCA循环彻底分解生物分子,以ATP的形式产生能量。多数情况下P53促进TCA循环。丙酮酸脱氢酶激酶(pyruvate dehydrogenase kinase,PDK)负向调节丙酮酸脱氢酶(pyruvate dehydrogenase,PDH),后者可将丙酮酸转化为乙酰辅酶A以进入TCA循环,而P53通过抑制PDK1/2来促进丙酮酸转化[27-28]。另外,在胰腺导管腺癌中,P53通过诱导异柠檬酸脱氢酶1 (isocitrate dehydrogenase 1,IDH1)和丙酮酸羧化酶(pyruvate carboxylase,PC)来激活TCA循环,导致α-酮戊二酸(α-ketoglutarate,α-KG)积累,P53缺失可调节a-KG依赖的染色质修饰酶的活性,从而影响癌细胞的表观基因组并使其恶化[29]。此外,作为TCA循环的重要物质来源,当葡萄糖不足时,谷氨酰胺可转化为谷氨酸,继而转化为α-KG,后者进入并维持TCA循环[30],P53通过激活谷氨酰胺酶2(glutaminase 2,GLS2)来放大此过程[20],GLS2以P53依赖的方式降低细胞对活性氧(reactive oxygen species,ROS)相关凋亡的敏感性[2],促进谷氨酰胺分解和OXPHOS。同时,P53通过抑制苹果酸酶1/2 (malic enzyme 1/2,ME1/2)[22]来抑制谷氨酰胺分解,以调节TCA循环活性,加速癌细胞衰老(图1)。PPP为细胞提供所需的核糖和还原型烟酰胺腺嘌呤二核苷酸磷酸(reduced nicotinamide adenine dinucleotide phosphate,NADPH),但P53在PPP中的作用是矛盾的。P53既能通过调节磷酸果糖激酶(phosphofructokinase, PFK)3/4和TIGAR来控制糖酵解通量和G6P进入PPP的水平从而促进PPP[4,7,31],又能直接结合葡萄糖-6-磷酸脱氢酶(glucose-6-phosphate dehydrogenase,G-6-PD)阻止其活性二聚体的形成或限制PFK4的表达来灭活G-6-PD,进而抑制PPP[17]。上述P53的不同作用取决于细胞类型和应激条件。
1.3 P53与OXPHOS和线粒体代谢
P53在大多数细胞中通过调节Warburg效应下游的靶基因促进OXPHOS。P53可诱导细胞色素C氧化酶2(synthesis of cytochrome C oxidase 2,SCO2)来增强线粒体OXPHOS[32],也能调控凋亡诱导因子(apoptosis-inducing factor,AIF)来维持线粒体电子传递链中复合物Ⅰ的完整性[33]。此外,P53调节线粒体转录因子A (mitochondrial transcription factor A,TFAM)和铁氧还蛋白还原酶(ferredoxin reductase,FDXR)来促进电子从NADPH转移到细胞色素p450上,进而促进OXPHOS。PARK2是P53的靶基因,介导线粒体自噬,清除受损线粒体。P53还可直接与线粒体铁转运蛋白SLC25A28结合,使铁异常积累、ROS生成增加、扰乱电子传递链活性,最终导致肝星状细胞铁中毒,改善肝纤维化损伤[34]。研究显示,P53能易位到线粒体上与寡霉素敏感相关蛋白(oligomycin sensitivity-conferring protein,OSCP)结合,促进F1F0-ATP合酶的组装[35],同时P53结合NF-κB亚基RelA抑制其线粒体易位,从而消除RelA介导的OXPHOS抑制[36]。ARTS是位于线粒体外膜的促凋亡蛋白,可被P53激活并与P53协同抑制Bcl-XL,以促进P53依赖的线粒体凋亡[37]。在许多癌症中,线粒体亚甲基四氢叶酸脱氢酶2(mitochondrial methylenetetrahydrofolate dehydrogenase 2, MTHFD2)的表达与P53高度相关,能使叶酸代谢增加、体内外肿瘤生长加快,而P53能下调MTHFD继而抑制肿瘤生长[38]。此外,P53通过控制线粒体的结构和动力学,促进动力相关蛋白1(dynamin-related protein 1,DRP1)的表达和线粒体融合蛋白1(L-optic atrophy 1,L-Opa1)的加工来支持线粒体分裂[39]。P53也可调控外膜转位酶20(translocase of outer membrane 20,TOM20)、内膜转位酶23(translocase of inner membrane 23,TIM23)及线粒体热激蛋白60/70(mitochondrial heat shock protein 60/ 70, mtHSP70/60)来促进线粒体代谢[22]。
2 P53与脂代谢
脂质是人体必需的三大宏量营养素之一,参与机体能量代谢和能量储存。正常细胞主要通过外周循环吸收来满足对脂肪的需求,表现出低水平的从头脂肪生成。多数肿瘤细胞倾向于促进新生脂质合成,并增加脂质摄取以满足快速增殖的需求,因此异常的脂质合成与代谢是癌细胞重要标志之一。P53能够调控这些效应,如在葡萄糖不足时,胍基乙酸甲基转移酶(guanidinoacetate methyltransferase,GAMT)促进肌酸生物合成和脂肪酸β-氧化(β-oxidation,FAO)以维持能量稳态,P53能调控GAMT的表达来调节脂肪代谢。
2.1 P53与脂质合成
P53在调控脂质合成中发挥重要作用,研究[40]证实P53抑制脂肪细胞分化和脂肪生成,如P53抑制ME2以阻碍脂肪生成[41]。甾醇调节元件结合蛋白-1(sterol regulatory element-binding protein-1,SREBP-1)是靶向脂肪生成基因的关键转录因子,能调节ATP柠檬酸裂解酶(ATP citrate lyase,ACLY)、脂肪酸合酶(fatty acid synthase,FASN)等参与脂肪酸合成的关键酶,Moon等[42]研究发现,P53可结合并抑制SREBP-1表达,进而抑制脂肪酸合成。Zhang等[43]研究发现,缺乏FDXR可促进SREBP-1/2活化并下调ATP转运结合子A1(ATP-binding cassette transporter A1,ABCA1)表达,使细胞胆固醇和三酰甘油水平升高,而FDXR-P53轴通过上调ABCA1来抑制SREBP-1/2的成熟及核转位,从而减少从头脂质合成。此外,P53通过G-6-PD抑制PPP来降低NADPH水平[2],进而减少脂肪生成(图2A)。在肝细胞中,P53激活骨桥蛋白(osteopontin,OPN)抑制衰老相关的三酰甘油合成[44]。P53还可以通过编码β3-肾上腺素能受体的基因促进脂肪分解[45]。
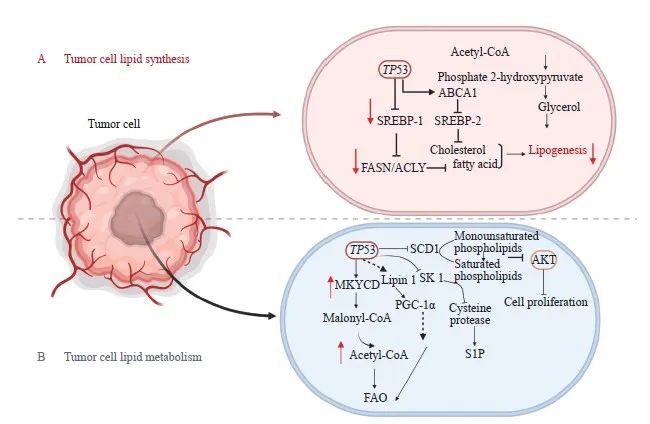
图2 P53调节肿瘤细胞中脂合成与代谢
Fig. 2 P53 regulates lipid synthesis and metabolism in tumor cells
A: P53 regulates lipid synthesis; B: P53 regulates lipid metabolism. P53 suppresses adipose differentiation and adipogenesis in tumor cells. P53 promotes FAO by activating MLYCD and PANK 1. P53 promotes FAO by activating MLYCD and PANK 1. P53 suppresses sphingosine kinases 1 to regulate S1P metabolism. Lipin 1: Homo sapiens lipin 1.
2.2 P53与FAO
FAO是机体能量来源之一,也是体内脂肪酸分解的主要途径,Wang等[46]研究发现P53促进FAO。P53通过激活丙二酰辅酶A脱羧酶(malonyl-CoA decarboxylase,MLYCD)和PANK1,提高线粒体乙酰辅酶A的含量并提供还原型黄素腺嘌呤二核苷酸(reduced flavin adenine dinucleotide,FADH2)和还原型烟酰胺腺嘌呤二核苷酸(reduced nicotinamide adenine dinucleotide,NADH),以维持电子传递链的活性,进而促进FAO[24,47]。P53激活Lpin1基因,使之与过氧化物酶体增殖物激活受体γ共激活因子1α(peroxisome proliferators-activated receptor γ coactivator-1α,PGC-1α)相互作用,充当核转录共激活物,间接增强FAO[48]。P53还通过抑制硬脂酰辅酶A去饱和酶1(stearyl coenzyme desaturation enzyme 1,SCD1)的表达,将单不饱和磷脂转变为饱和磷脂,进而抑制致癌蛋白激酶B(protein kinase B,AKT)通路并阻碍肿瘤生长[49]。
2.3 P53与鞘脂代谢和乙醇代谢
P53在鞘脂代谢中发挥关键作用,可以通过抑制鞘氨醇激酶1(sphingosine kinases 1,SK1)来调控鞘氨醇-1-磷酸(sphingosine-1-phosphate,S1P)的代谢[50],Jiao等[51]在TP53基因敲除的小鼠中发现,失活的SK1能够消除淋巴瘤的形成(图2B)。Chen等[52]研究发现,P53诱导脱氢酶3(dehydrogenase 3,DHRS3)表达,促进脂滴形成和脂质堆积。但在营养物质不足时,P53通过调节芳香化酶和SIRT1来抑制肝细胞的脂质积累[22,43]。
P53在乙醇性脂肪性肝病、脂肪性肝炎和肝细胞癌的发展中也发挥作用[53]。Yao等[54]研究发现,P53通过两种机制下调乙醛脱氢酶2(aldehyde dehydrogenase 2,ALDH2)来抑制乙醇代谢,进而预防乙醇性脂肪肝:一是P53直接与ALDH2结合,阻止其二聚体或四聚体形成;二是P53降低丙酮酸水平以抑制ALDH2的激活。但抑制ALDH2可能导致机体内乙醛堆积而造成对多种癌症的易感性。然而,由于P53始终保持较低水平,因此不会显著增加正常细胞或组织中的乙醛含量和对癌症的易感性。
3 P53与核苷酸代谢
P53在调节核苷酸代谢中发挥重要作用,有助于抑制癌细胞增殖。P53可以通过抑制核糖核苷酸还原酶亚基1/2的表达,来抑制核苷酸合成[55]。此外,P53通过抑制脱氧尿苷焦磷酸酶(deoxyuridine 5’-triphosphate nucleotidohydrolase,dUTPase)和鸟苷酸合成酶(guanosine monophosphate synthetase,GMPS)分别抑制脱氧胸苷三磷酸(deoxythymidine triphosphate,dTTP)和鸟苷一磷酸(guanosine monophos-phate,GMP)的合成。P53还通过诱导miR-34a来抑制程序性死亡[蛋白]配体-1(programmed death ligand-1,PD-L1)的表达,间接破坏肌苷单磷酸脱氢酶1(inosine monophosphate dehydrogenase 1,IMPDH1)的翻译,从而抑制鸟苷三磷酸(guanosine triphosphate,GTP)合成[1]。同时,P53诱导TIGAR或抑制PFK3以应答DNA损伤,并促进DNA损伤后的核苷酸合成[56]。值得注意的是,ATP/二磷酸腺苷(adenosine diphosphate,ADP)可以直接调控P53与其DNA靶标间的结合[57],或通过AMPK和mTOR通路间接影响P53的稳定性和活性。此外,P53可诱导膜腺苷A2b受体(adenosine A2B receptor,ADORA2b)的表达以激活下游凋亡通路,进而抑制肿瘤[58]。Rather等[59]提出NADP通过G-6-PD和ME迅速转化为NADPH,后者在p53突变的癌细胞中堆积以增加核苷酸的合成。
4 P53与氨基酸代谢
癌细胞对某些氨基酸特别是丝氨酸的需求显著增加,丝氨酸代谢在癌症中经常失调[60-61]。磷酸甘油酸脱氢酶(phosphoglycerate dehydrogenase,PHGDH)是丝氨酸合成的关键酶,在促进肿瘤发生中发挥重要作用,Shi等[62]研究发现,P53下调PHGDH表达来抑制细胞中丝氨酸的从头合成。Song等[63]研究表明,丝氨酸不足时,结直肠癌细胞中的P53会激活p21介导的短暂G1细胞周期停滞,促进癌细胞存活,相反,P53-/-结直肠癌细胞不能完成对丝氨酸饥饿的应答,导致氧化应激、生存力降低和增殖受损。P53分别PHGDH和胱硫氨酸β-合成酶(cystathionine β-synthetase,CBS)来抑制丝氨酸合成途径和转硫化途径,从而限制谷胱甘肽的产生[34]。在P53+/+癌组织中,高表达的SIRT通过抑制谷氨酰胺代谢促进P53磷酸化,抑制肿瘤发展[2]。P53诱导GLS2表达来调节谷氨酰胺代谢,如在葡萄糖不足时,P53活化GLS2使细胞继续产能,以维持细胞存活和生长[64]。SLC1A3是维持电子传递链和TCA活性的关键,在谷氨酰胺不足时,P53上调SLC1A3表达使谷氨酸、谷氨酰胺和核苷酸重新合成[65],促进细胞利用天门冬氨酸,以恢复细胞活力进而满足细胞需求。
5 展望
为满足肿瘤增殖时急剧增加的物质和能量需求,癌细胞提高了对营养物质的摄取,以确保最大限度地利用糖酵解和OXPHOS的代谢中间体。P53不仅在细胞周期阻滞或细胞凋亡中发挥重要作用,而且参与多种生理和病理学过程的调节。不论是抑制肿瘤还是维持正常细胞内稳态,P53的多重功能越来越受到重视。
当细胞应激时,P53会通过调控葡萄糖、脂肪酸、氨基酸和核苷酸来影响各种代谢途径。癌细胞更倾向于糖酵解而不是OXPHOS来产生中间代谢物和能量,在多数细胞中,P53可以从多个步骤促进OXPHOS,并抑制糖酵解,从而抑制癌细胞生长。P53介导的糖酵解抑制有助于糖异生和PPP,然而也有报道称P53通过某些通路抑制糖异生和PPP。Liu等[22]解释了该矛盾,一方面, P53可以降低外界对细胞的干扰,如轻度DNA损伤时,P53促进DNA修复以避免损伤扩大而促进细胞生存;另一方面,P53会引起细胞衰老、凋亡、坏死等,该机制可以淘汰损伤的细胞。因此,二者在一定程度上都可以抑制肿瘤;P53还能促进TCA和线粒体代谢。此外,P53通过多种方式抑制脂质合成,并促进FAO。最后,P53被证明通过多种途径抑制癌细胞的核苷酸和氨基酸合成。P53在代谢上的作用相互交织,通过提高对P53相关代谢网络的认识,有望找到新的肿瘤治疗靶点。
利益冲突声明:所有作者均声明不存在利益冲突。
[参考文献]
[1]LACROIX M, RISCAL R, ARENA G, et al. Metabolic functions of the tumor suppressor p53: implications in normal physiology, metabolic disorders, and cancer[J]. Mol Metab, 2020, 33: 2-22.
[2]MAO Y, JIANG P. The crisscross between p53 and metabolism in cancer[J]. Acta Biochim Biophys Sin (Shanghai), 2023, 55(6): 914-922.
[3]FU X, WU S, LI B, et al. Functions of p53 in pluripotent stem cells[J]. Protein Cell, 2020, 11(1): 71-78.
[4]ZAFAR A, KHAN M J, NAEEM A. MDM2- an indispensable player in tumorigenesis[J]. Mol Biol Rep, 2023, 50(8): 6871-6883.
[5]HOU Y C, ZHANG X T, YAO H, et al. METTL14 modulates glycolysis to inhibit colorectal tumorigenesis in p53-wild-type cells[J]. EMBO Rep, 2023, 24(4): e56325.
[6]MARCUCCI F, RUMIO C. On the role of glycolysis in early tumorigenesis-permissive and executioner effects[J]. Cells, 2023, 12(8): 1124.
[7]SHEN J Z, WANG Q R, MAO Y N, et al. Targeting the p53 signaling pathway in cancers: molecular mechanisms and clinical studies[J]. MedComm (2020), 2023, 4(3): e288.
[8]WHITT A G, NEELY A M, SARKAR O S, et al. Paraoxonase 2 (PON2) plays a limited role in murine lung tumorigenesis[J]. Sci Rep, 2023, 13(1): 9929.
[9]KLEINEHR J, SCHÖFBÄNKER M, DANIEL K, et al. Glycolytic interference blocks influenza A virus propagation by impairing viral polymerase-driven synthesis of genomic vRNA[J]. PLoS Pathog, 2023, 19(7): e1010986.
[10]MUKHERJEE A G, GOPALAKRISHNAN A V. The mechanistic insights of the antioxidant Keap1-Nrf2 pathway in oncogenesis: a deadly scenario[J]. Med Oncol, 2023, 40(9): 248.
[11]EWUNKEM A J, DEVE M, HARRISON S H, et al. Diepoxybutane induces the p53-dependent transactivation of the CCL4 gene that mediates apoptosis in exposed human lymphoblasts[J]. J Biochem Mol Toxicol, 2023, 37(5): e23316.
[12]HUANG J J, DU J J, LIN W J, et al. Regulation of lactate production through p53/β-enolase axis contributes to statin-associated muscle symptoms[J]. EBioMedicine, 2019, 45: 251-260.
[13]CHETTA P, SRIRAM R, ZADRA G. Lactate as key metabolite in prostate cancer progression: what are the clinical implications? [J]. Cancers (Basel), 2023, 15(13): 3473.
[14]KAM C S, HO D W, MING V S, et al. PFKFB4 drives the oncogenicity in TP53-mutated hepatocellular carcinoma in a phosphatase-dependent manner[J]. Cell Mol Gastroenterol Hepatol, 2023, 15(6): 1325-1350.
[15]KOUZU H, TATEKOSHI Y, CHANG H C, et al. ZFP36L2 suppresses mTORc1 through a P53-dependent pathway to prevent peripartum cardiomyopathy in mice[J]. J Clin Invest, 2022, 132(10): e154491.
[16]ROBERSON P A, KINCHELOE G N, WELLES J E, et al. Glucose-induced activation of mTORC1 is associated with hexokinase 2 binding to sestrins in HEK293T cells[J]. J Nutr, 2023, 153(4): 988-998.
[17]CASTELLANOS G, VALBUENA D S, PÉREZ E, et al. Chromosomal instability as enabling feature and central hallmark of breast cancer[J]. Breast Cancer (Dove Med Press), 2023, 15: 189-211.
[18]WANG H L, GUO M, WEI H D, et al. Targeting p53 pathways: mechanisms, structures, and advances in therapy[J]. Signal Transduct Target Ther, 2023, 8(1): 92.
[19]ZHAN H, ZHANG Q, ZHANG C, et al. Targeted activation of HNF4α by AMPK inhibits apoptosis and ameliorates neurological injury caused by cardiac arrest in rats[J]. Neurochem Res, 2023, 48(10): 3129-3145.
[20]XIONG C, LING H, HAO Q, et al. Cuproptosis: P53-regulated metabolic cell death? [J]. Cell Death Differ, 2023, 30(4): 876-884.
[21]TANG M, XU H, HUANG H Y, et al. Metabolism-based molecular subtyping endows effective ketogenic therapy in p53-mutant colon cancer[J]. Adv Sci (Weinh), 2022, 9(29): e2201992.
[22]LIU Y Q, GU W. The complexity of p53-mediated metabolic regulation in tumor suppression[J]. Semin Cancer Biol, 2022, 85: 4-32.
[23]HAN Y L, LIANG C, YU Y X, et al. Gluconeogenesis alteration and p53-SIRT6-Fox01 signaling adaptive regulation in sheep from different grazing periods[J]. Comput Math Methods Med, 2022, 2022: 4614665.
[24] SANFORD J D, FRANKLIN D, GROIS G A, et al. Carnitine o-octanoyltransferase is a p53 target that promotes oxidative metabolism and cell survival following nutrient starvation[J]. J Biol Chem, 2023, 299(7): 104908.
[25] ZHANG X Y, TAO G R, JIANG J, et al. PCK1 activates oncogenic autophagy via down-regulation serine phosphorylation of UBAP2L and antagonizes colorectal cancer growth[J]. Cancer Cell Int, 2023, 23(1): 68.
[26] REINISCH I, KLYMIUK I, MICHENTHALER H, et al. p53 regulates a miRNA-fructose transporter axis in brown adipose tissue under fasting[J]. Front Genet, 2022, 13: 913030.
[27] SAFI A, SABERIYAN M, SANAEI M J, et al. The role of noncoding RNAs in metabolic reprogramming of cancer cells[J]. Cell Mol Biol Lett, 2023, 28(1): 37.
[28] LIU Y, WANG J D, JIANG M X. Copper-related genes predict prognosis and characteristics of breast cancer[J]. Front Immunol, 2023, 14: 1145080.
[29] MORRIS J P 4th, YASHINSKIE J J, KOCHE R, et al. Alphaketoglutarate links p53 to cell fate during tumour suppression[J]. Nature, 2019, 573(7775): 595-599.
[30] DA FONSECA JUNIOR A M, ISPADA J, DOS SANTOS E C, et al. Adaptative response to changes in pyruvate metabolism on the epigenetic landscapes and transcriptomics of bovine embryos[J]. Sci Rep, 2023, 13(1): 11504.
[31] LI X, WU L M, ZOPP M, et al. p53-TP53-induced glycolysis regulator mediated glycolytic suppression attenuates DNA damage and genomic instability in fanconi anemia hematopoietic stem cells[J]. Stem Cells, 2019, 37(7): 937-947.
[32] WEI J, WANG S, ZHU H, et al. Hepatic depletion of nucleolar protein mDEF causes excessive mitochondrial copper accumulation associated with p53 and NRF1 activation[J]. iScience, 2023, 26(7): 107220.
[33] JAHOOR ALAM M. Insights from the p53 induced TIGAR protein 2 in the glycolytic pathway model[J]. Bioinformation, 2022, 18(3): 310-317.
[34] LIU Y Q, GU W. p53 in ferroptosis regulation: the new weapon for the old guardian[J]. Cell Death Differ, 2022, 29(5): 895-910.
[35] DOU X Z, GUO H, D’AMICO T, et al. CryoEM structure with ATP synthase enables late-stage diversification of Cruentaren A[J]. Chemistry, 2023, 29(29): e202300262.
[36] ROHBECK E, NIERSMANN C, KÖHRER K, et al. Positive allosteric GABAA receptor modulation counteracts lipotoxicityinduced gene expression changes in hepatocytes in vitro[J]. Front Physiol, 2023, 14: 1106075.
[37] HAO Q, CHEN J X, LU H, et al. The ARTS of p53-dependent mitochondrial apoptosis[J]. J Mol Cell Biol, 2023, 14(10): mjac074.
[38] LI G, WU J, LI L, et al. p53 deficiency induces MTHFD2 transcription to promote cell proliferation and restrain DNA damage[J]. Proc Natl Acad Sci USA, 2021, 118(28): e2019822118.
[39] RAINHO M A, SIQUEIRA P B, DE AMORIM Í S S, et al. Mitochondria in colorectal cancer stem cells-a target in drug resistance[J]. Cancer Drug Resist, 2023, 6(2): 273-283.
[40] ZHANG K X, YANG X H, ZHENG M Y, et al. Acetylated- PPARγ expression is regulated by different p53 genotypes associated with the adipogenic differentiation of polyploid giant cancer cells with daughter cells[J]. Cancer Biol Med, 2023, 20(1): 56-76.
[41] LI W, KOU J J, ZHANG Z X, et al. Cellular redox homeostasis maintained by malic enzyme 2 is essential for MYC-driven T cell lymphomagenesis[J]. Proc Natl Acad Sci U S A, 2023, 120(23): e2217869120.
[42] MOON S H, HUANG C H, HOULIHAN S L, et al. p53 represses the mevalonate pathway to mediate tumor suppression[J]. Cell, 2019, 176(3): 564-580.e19.
[43] ZHANG Y H, MOHIBI S, VASILATIS D M, et al. Ferredoxin reductase and p53 are necessary for lipid homeostasis and tumor suppression through the ABCA1-SREBP pathway[J]. Oncogene, 2022, 41(12): 1718-1726.
[44] GÓMEZ-SANTOS B, SAENZ DE URTURI D, NUÑEZGARCÍA M, et al. Liver osteopontin is required to prevent the progression of age-related nonalcoholic fatty liver disease[J]. Aging Cell, 2020, 19(8): e13183.
[45] KANG J G, LAGO C U, LEE J E, et al. A mouse homolog of a human TP53 germline mutation reveals a lipolytic activity of p53[J]. Cell Rep, 2020, 30(3): 783-792.e5.
[46] WANG C Y, WANG C H, MAI R T, et al. Mutant p53- microRNA-200c-ZEB2-axis-induced CPT1C elevation contributes to metabolic reprogramming and tumor progression in basal-like breast cancers[J]. Front Oncol, 2022, 12: 940402.
[47] XU R, WANG W N, ZHANG W L. Ferroptosis and the bidirectional regulatory factor p53[J]. Cell Death Discov, 2023, 9(1): 197.
[48] KUO H C, LUO L X, MA Y, et al. The p53 transactivation domain 1-dependent response to acute DNA damage in endothelial cells protects against radiation-induced cardiac injury[J]. Radiat Res, 2022, 198(2): 145-153.
[49] THIBAULT B, RAMOS-DELGADO F, GUILLERMETGUIBERT J. Targeting class Ⅰ-Ⅱ-Ⅲ PI3Ks in cancer therapy: recent advances in tumor biology and preclinical research[J]. Cancers (Basel), 2023, 15(3): 784.
[50] GAO Y Q, JIAO Y T, GONG X Y, et al. Role of transcription factors in apoptotic cells clearance[J]. Front Cell Dev Biol, 2023, 11: 1110225.
[51] JIAO Z, PAN Y, CHEN F. The metabolic landscape of breast cancer and its therapeutic implications[J]. Mol Diagn Ther, 2023, 27(3): 349-369.
[52] CHEN M, CHEN Y. Bioinformatics analysis of common genetic and molecular traits and association of portal hypertension with pulmonary hypertension[J]. J Healthc Eng, 2022, 2022: 9237701.
[53]RYBICKA M, VERRIER E R, BAUMERT T F, et al. Polymorphisms within DIO2 and GADD45A genes increase the risk of liver disease progression in chronic hepatitis b carriers[J]. Sci Rep, 2023, 13(1): 6124.
[54]YAO P B, ZHANG Z X, LIU H C, et al. P53 protects against alcoholic fatty liver disease via ALDH2 inhibition[J]. EMBO J, 2023, 42(8): e112304.
[55]XU L, CHEN Z J, ZHANG Y, et al. P53 maintains gallid alpha herpesvirus 1 replication by direct regulation of nucleotide metabolism and ATP synthesis through its target genes[J]. Front Microbiol, 2022, 13: 1044141.
[56]CHEN S M, DUAN Y M, WU Y H, et al. A novel integrated metabolism-immunity gene expression model predicts the prognosis of lung adenocarcinoma patients[J]. Front Pharmacol, 2021, 12: 728368.
[57]KEALEY J, DÜSSMANN H, LLORENTE-FOLCH I, et al. Effect of TP53 deficiency and KRAS signaling on the bioenergetics of colon cancer cells in response to different substrates: a single cell study[J]. Front Cell Dev Biol, 2022, 10: 893677.
[58]JACOBERGER-FOISSAC C, COUSINEAU I, BARECHE Y, et al. CD73 inhibits cGAS-STING and cooperates with CD39 to promote pancreatic cancer[J]. Cancer Immunol Res, 2023, 11(1): 56-71.
[59]RATHER G M, PRAMONO A A, SZEKELY Z, et al. In cancer, all roads lead to NADPH[J]. Pharmacol Ther, 2021, 226: 107864.
[60]MIKI K, YAGI M, NOGUCHI N, et al. Induction of glioblastoma cell ferroptosis using combined treatment with chloramphenicol and 2-deoxy-D-glucose[J]. Sci Rep, 2023, 13(1): 10497.
[61]WANG K, LUO L, FU S Y, et al. PHGDH arginine methylation by PRMT1 promotes serine synthesis and represents a therapeutic vulnerability in hepatocellular carcinoma[J]. Nat Commun, 2023, 14(1): 1011.
[62]SHI T Z, YUAN Z H, HE Y Y, et al. Competition between p53 and YY1 determines PHGDH expression and malignancy in bladder cancer[J]. Cell Oncol (Dordr), 2023: 46(5): 1457-1472.
[63]SONG X M, CHEN Q, WANG J F, et al. Clinical and prognostic implications of an immune-related risk model based on TP53 status in lung adenocarcinoma[J]. J Cell Mol Med, 2022, 26(2): 436-448.
[64]ZAVILEYSKIY L, BUNIK V. Regulation of p53 function by formation of non-nuclear heterologous protein complexes[J]. Biomolecules, 2022, 12(2): 327.
[65]INDEGLIA A, LEUNG J C, MILLER S A, et al. An African-specific variant of TP53 reveals PADI4 as a regulator of p53-mediated tumor suppression[J]. Cancer Discov, 2023, 13(7): 1696-1719.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言











#p53# #糖代谢# #氨基酸代谢# #脂肪酸氧化# #核苷酸代谢#
35
新的认识,黑暗中的灯塔!!!!!
24